ASTM D7995-19
Standard Test Method for Total Water in Liquid Butane by Liquefied Gas Sampler and Coulometric Karl Fischer Titration
This test method describes the use of a specialized liquefied gas sampler coupled to a coulometric Karl Fischer (KF) titrator for the determination of water in liquid butane with water concentrations from 1 mg/kg to 100 mg/kg.
NOTE 1: Other liquefied petroleum gases described in Specification D1835 including propane, propene (propylene), butylenes and mixtures of these materials and other light hydrocarbons, and dimethyl ether described in Specification D7901, can be analyzed by this method but the precision has not been studied and therefore the stated precision has not been validated for these materials.
ASTM has developed more than 25 Standard Test Methods for the determination of moisture in a Wide range of products and materials utilizing the Karl Fischer reaction. The accurate determination of water content in Liquified Petroleum Gases is vital to quality assessment, quality control, refining, handling, transportation and sales.
Water should be controlled to avoid freeze ups of pressure reducing and similar equipment when dissolved water separate from the product and freeze.
Water will also form hydrates in pipelines causing operational problems due to accumulation of granular solids or slushy substances.
Referenced Documents
ASTM Standards:
D1265 Practice for Sampling Liquified Petroleum (LP) Gases, Manual Method
D1835 Specifications for Liquified Petroleum (LP) Gases
D2713 Test Method for Dryness of Propane (Valve Freeze Method)
D3700 Practice for Obtaining LPG Samples Using a Floating Piston Cylinder
D5623 Test Method for Sulfur Compounds in Light Petroleum Liquids by Gas Chromatography and Sulfur Selective Detection
D6299 Practice for Applying Statistical Quality Assurance and Control Charting Techniques to Evaluate Analytical Measurement System Performance
D6300 Practice for Determination of Precision and Bias Data for Use in Test Methods for Petroleum Products and Lubricants
D6304 Test Method for Determination of Water in Petroleum Products, Lubricating Oils and Additives by Coulometric Karl Fischer Titration
D7901 Specifications for Dimethyl Ether for Fuel Purposes
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E203 Test Method for Water Using Volumetric Karl Fischer Titration
Terminology
Definitions:
liquefied petroleum gas, (LP Gas, LPG), n—a narrow boiling range mixture of hydrocarbons consisting of propane, propylene, butanes and butylenes, individually or in specified combinations, with limited amounts of other hydrocarbons and naturally occurring non-hydrocarbons.
dimethyl ether (DME), n—the chemical compound CH3OCH3.
Definitions of Terms Specific to This Standard:
coulometric Karl Fischer titration (coulometric titration), n—in reference to Karl Fischer titration methods, a process of measuring the water content of a sample using an electrolytic process to generate iodine in situ.
mass flow meter, n—a device used to measure the flow of gases.
Abbreviations:
DME—dimethyl ether
H2S—hydrogen sulfide
KF—Karl Fischer (titration)
LPG—liquefied petroleum gas
MFM—mass flow meter
QA—quality assurance
QC—quality control
Summary of Test Method
An aliquot of pressurized liquid butane sample is introduced into the liquefied gas sampler, where the sample is totally volatilized and passes through a heated chamber (typically 60 °C to 80 °C) to ensure the sample is in a gaseous state. The gas then flows through a calibrated mass flow meter (MFM) or a fixed volume sample loop and bubbles into the electrolytic cell of a coulometric Karl Fischer (KF) titrator. The MFM is calibrated to the sample composition analyzed and a calibration factor is used to calculate the mass of pressurized sample. Alternatively, when a fixed volume sample loop is used, the temperature and the pressure are measured to calculate the volume of the volatized sample gas. The gas flow is stopped when a suitable amount of sample is introduced to the coulometric KF titrator. The water in the gas is absorbed by the anode reagent and titrated automatically by the coulometric KF titrator. The concentration of water in the original pressurized sample is calculated by the amount of water measured (µg) and the sample size (g) introduced by the MFM or sample loop.
General Descriptions of Volumetric Liquefied Gas Sampler—There are two types of liquefied gas sampler. The first utilizes a mass flow meter to accurately measure the mass or volume of liquefied gas is introduced into a KF titration cell, see Fig. 1. Once the specified mass or volume of sample is delivered, the sampler automatically stops the flow into the titration cell. The second type of sampler uses a fixed volume sample loop to accurately deliver an aliquot of liquefied gas into the titration cell, see Fig. 2. The fixed volume is carefully delivered into the KF titration cell by means of a restrictor valve. In the coulometric KF titration technique, the sample is introduced into an electrolytic cell where the iodine required for the reaction with water is produced by an anodic oxidation of iodide. With the coulometric KF titration technique, no standardization of reagents is required.
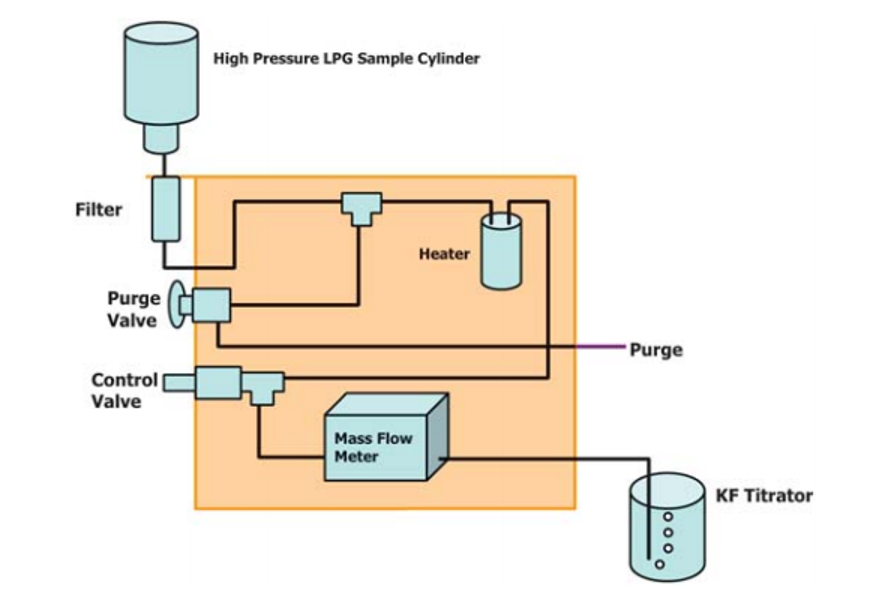
Figure 1, Typical Block Flow Diagram of LPG Sampler Using MFM
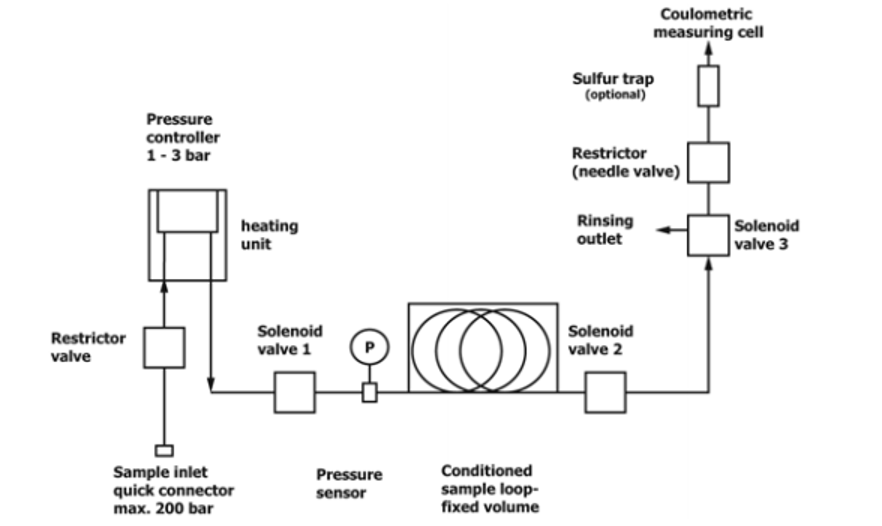
Figure 2. Typical Block Diagram of LPG Sampler with Fixed Volume Sample Loop
Significance and Use
High water concentrations can have a detrimental effect on the many uses of liquefied petroleum gas (LPG). Wet butane, propane, and other low molecular weight hydrocarbon products can cause operational issues in customer equipment and downstream processes. Water can cause corrosion problems and create safety hazards during the storage, distribution and use of liquefied petroleum gas (LPG) and pressurized low molecular weight hydrocarbon samples.
5.2 While the dryness of propane may be monitored with a functional” test such as the valve freeze Test Method D2713, this test method provides an analytical method to directly measure water content in LPG and pressurized low molecular weight hydrocarbons and their mixtures.
Apparatus
Liquefied Gas Sampler—An apparatus that automatically controls the introduction of liquefied gas sample from a sample cylinder into the electrolytic titration cell of a coulometric Karl Fischer (KF) titrator as specified below. The amount of the volatilized sample introduced into the titration cell is based on the type of liquefied gas sampler being used. A typical block flow diagram of the types two of liquefied gas samplers are shown in Fig. 1 and Fig. 2.
Liquefied Gas Sample Utilizing a Mass Flow Meter— The pressurized sample is introduced into the sampler and vaporized at 60 °C to 80 °C. Sample flow is controlled with a needle valve and monitored with a built-in MFM calibrated to the sample matrix being analyzed (see Section 14 D7995). The flow of sample into the titration vessel is stopped when the set amount of sample has been introduced into the titration vessel and is then analyzed.
Liquefied Gas Sample Utilizing a Fixed Sample Loop—The pressurized sample is introduced into the sample and vaporized at 60 °C to 80 °C and flows into the fixed sample loop. The sample loop is filled and is introduced into the titration vessel. The amount of sample introduced into the titration vessel is automatically calculated based on the volume of the built-in sample loop, temperature, and pressure at the time of analysis.
The liquefied gas sampler may be programmed to automatically sample and analyze multiple replicates from the same pressurized sample cylinder with no operator intervention.
Balance—A balance is required to determine the calibration factor of each type of gas passing through the mass flow meter. To achieve a sufficient level of accuracy, the sample weight difference should have at least three significant figures for calibration. It is recommended the balance have accuracy to one hundredth of a gram.
Coulometric Automatic Titrator–, consisting of a control unit, titration vessel, dual platinum sensing electrode, generator electrode assembly, and magnetic stirrer. The instrument is designed to coulometrically generate iodine that reacts stoichiometrically with the water present in the sample solution. The coulombs of electricity required to generate iodine are converted to micrograms of water, which is obtained as a direct digital readout.
Figure 1. Aquamax Pro LPG Karl Fischer Titrator
Interferences
Certain compounds or classes of compounds interfere with the accurate determination of water by the Karl Fischer test method; including aldehydes, ketones, free halogens, ferric salts, and strong oxidizing and reducing agents.
In LPG, the most common interferences are mercaptan sulfur and hydrogen sulfide by means of the reactions in Eq 1 and 2. In commercial butane, propane, and LPG, ethyl mercaptan is commonly used as an odorant. For propane, the standard practice is to add 1.5 lb of ethyl mercaptan to 10 000 gal of propane which equates to 25 ppm by volume or 35 mg ⁄kg ethyl mercaptan.
2R-SH + I2 → RS-SR + 2HI (1)
where: R-SH = mercaptan, and
RS-SR = organic disulfide.
H2S + I2 → S + 2HI (2)
If the concentration of mercaptans in the samples is expected to be above 35 mg ⁄kg or the interference from mercaptan concentrations in the sample will result in significant interference relative to the expected amount of water in the samples being analyzed, refer to Appendix X2 of D7995 for handling of mercaptan interference.
Free halogens can oxidize the iodide in the KF reagent to form iodine and cause erroneously low water values.
A more detailed discussion of KF interferences can be found in Test Method E203 and other sources such as Mitchell, J. Jr. and Smith, D. M., Aquametry; A treatise on Methods for the Determination of Water, Part III–The Karl Fischer Reagent, 2nd ed., J. Wiley and Sons, Inc., New York, NY, 1977.
Reagents and Materials
Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the committee on Analytical Reagents of the American Chemical Society, where such specifications are available.6 Other grades may be used, provided it is pure enough to be used without lessening the accuracy of the determination.
Certified Water Standard—A certified standard solution that contains 0.1% water dissolved in an appropriate solvent. Commercial certified water standards are acceptable for use.
Certified Gas Standard—A certified standard gas that contains a definite water concentration (<100 mg/kg). Commercial certified water in gas standards based on nitrogen, methane, or LPG are acceptable for use.
Anode Reagent, for dual chamber (Fritted or Diaphragm) titration use reagent recommended by manufacturer of titrator.
Cathode Reagent, for dual chamber (Fritted or Diaphragm) titration use reagent recommended by manufacturer of titrator.
Single Chamber Reagent—Use single chamber (Fritless or Diaphragm Free) reagent recommended by manufacturer of titrator.
NOTE 2—Pyridine was the organic amine that was traditionally used in Karl Fischer reagents; however, pyridine-free formulations from various manufacturers are now available and preferred by most KF instrument manufacturers. Pyridine-free reagents are less toxic, less odorous, and more stable than pyridine-based reagents.
Methanol, dry, water content < 150 mg ⁄kg may be used to maintain constant volume of titration cell due to reagent volume loss by evaporation.
Inert Gas (Nitrogen, Helium or Argon), minimum 99.999 % purity, used to pressurize the sample cylinders prior, to and after analysis.
Gas-tight Luer-Lock Syringe, fitted with a cannula needle of appropriate length and gauge for introducing water standard into the titration chamber or removing excess solution from titration chamber. The volume of the syringe will depend on the sample size. Sample should occupy at least 25 % of the syringe volume.
It is recommended to rinse all glass syringes and needles with dry methanol or ethanol after cleaning, and then dry in an oven at 100 °C for at least 1 h and store in a desiccator.
Quality Guidelines
Autotitrators vary in calibration procedures by manufacturer. Consult the operating manual for the autotitrator in use. Stable, prepackaged Quality Control (QC) water standards are commercially available with 0.1 % by mass water content for this purpose. It is desirable to verify calibration with a standard solution that approximates the same range of water expected to be in the samples.
Certified water in gas standards are commercially available and may be used to verify system functionality.
It is recommended that a control chart measuring a QC standard sample be established and maintained according to generally accepted guidelines. Practice D6299 may be used for this purpose. Measure the titration control sample each day samples are tested. If the measured value exceeds ±10 % of the known amount, take appropriate action before proceeding with the sample test.
Hazards
Consult suppliers’ Safety Data Sheets (SDS) for materials used in this test method as well as any appropriate OSHA or equivalent USA or international safety rules and regulations.
Liquid butane, LPGs, and low molecular weight hydrocarbons gases under high pressures occur in the test method. Use cylinders and materials that are rated for containing these pressurized gases in all sample containers, tubing, and sample transfer apparatus that are exposed to these high pressures.
LPG and pressurized low molecular weight hydrocarbons and their volatilized gases are extremely flammable. The gas vapors generated during the sampling and purging process of the liquefied gas sampler shall be safely vented to avoid potential fire and explosion conditions to occur.
Sampling, Test Specimens and Test Units
Obtain a pressurized test sample in accordance with Practice D1265 or D3700. Since LPG normally contains a low concentration of water, ensure that sampling equipment is dry and is not exposed to atmospheric moisture. Sampling equipment may be dried with a dry inert gas.
When running replicate analyses from the same cylinder, ensure there is enough sample to support the number of replicates to be analyzed. See Table 1 for estimated sample amounts required based on expected water content.
It is recommended to pressurize all sample cylinders to the same pressure, nominally 1300 kPa to 2750 kPa (200 psi to 400 psi), with an inert gas (8.6) and maintain sufficient pressure in the sample cylinder during the analytical process to ensure representative and consistent samples for the number of replicate analyses being performed.
TABLE 1 Recommended Sample Size versus Expected Water Concentration
Expected Water Concentration (mg/kg) Sample Size (g)
0 to 50 1.5 to 10.0
50 to 150 0.5 to 5.0
Preparation of Apparatus
Follow the manufacturer’s directions for preparation and operation of the titration apparatus.
The recommended amount of coulometric reagents added to the electrolytic titration cell of a coulometric KF titrator usually has the capacity to react with up to 1000 mg of water. Replace these reagents when they are depleted by following manufacturer’s instructions.
Coulometric reagents are hygroscopic and shall be stored in tightly capped containers to reduce the absorption of atmospheric moisture.
Coulometric titration vessel should remain sealed to prevent introduction of atmospheric moisture that will decrease reagent life. Follow manufacturer’s recommendations for maintenance of the titration vessel.
Due to evaporation, dry methanol (as defined in Reagents and Materials) may be added to the titration vessel to maintain a constant volume. Replace according to manufacturer’s directions.
Ensure that the liquefied gas sampler is properly vented. Follow the manufacturer’s recommendations and requirements for proper venting of the liquefied gas sampler.
Verification of Instrument Accuracy
Daily operational checks of the entire system are recommended. This may be done by analyzing one or more of the following:
A gas standard containing a certified amount of water in LPG, nitrogen, or methane. A concentration of 25 mg ⁄kg of water is recommended.
A water standard containing a certified amount of water dissolved in an appropriate solvent.
Verification with Certified Gas Standard—Pass a suitable volume through the liquefied gas sampler and into the KF cell where it is titrated. The volume of sample is converted into a mass that is used to calculate water content. The amount of water determined is compared with the certified (theoretical) amount of water stated by the manufacturer. The deviation from the certified (theoretical) value shall not exceed 610 %.
Verification with Certified Water Solution—Inject a known volume or mass of a certified standard solution (8.2) directly into the KF vessel, determine the amount of water, and compare with the certified amount of water stated by the manufacturer. The deviation from the certified value shall not be larger than 65 %. A 0.1 % water standard is recommended. This serves as a verification of the titration but not the sample introduction mechanism.
Mass Flow Meter—Instruments that use a mass flow meter utilize a calibration factor for various sample matrices. The calibration factor is the ratio of the volume of gas allowed to flow through the mass flow meter and the corresponding mass loss of the sample cylinder. The procedure to obtain the calibration factor of the mass flow meter to multiple sample matrices being analyzed will vary between instrument manufacturers. It is recommended to check the calibration factor of the mass flow meter a minimum of once per year for each type of gas analyzed. Follow the manufacturer’s instruction regarding calibration of the mass flow meter.
Instruments with a fixed volume sample loop do not require a calibration of a MFM but require measurement of pressure and temperature to quantify the amount of sample introduced into the KF titration cell. Consult with the manufacturer for proper measurement of temperature and pressure.
Determination of the Calibration Factor for Mass Flow Meter
The calibration factor is used to convert volume of gas into mass of gas for each gas type for liquefied gas sampler utilizing mass flow meters.
Set up the coulometric KF titrator and liquefied gas sampler according to the manufacturer’s instructions and add the proper number of reagents.
Follow the manufacturer’s instructions for drying or conditioning the electrolytic cell prior to the start of the analysis.
Program the liquefied gas sampler following the manufacturer’s recommendations. Table 2 outlines recommended parameters for three different manufacturers of liquefied gas samplers.
Enter the volume of pressurized sample to be introduced into the KF titration cell into the appropriate field of the instrument software. In order to achieve sufficient accuracy, the sample weight difference should have at least three significant figures. One to ten liters of sample is recommended for calibration.
Place the sample cylinder on the balance and begin the calibration. Tare the balance and open the sample cylinder to begin the calibration.
After the minimum sample volume has passed through the MFM, close the sample valve and allow the remaining sample to enter the titration vessel. The water in the sample will be titrated during the calibration to maintain a dry titration cell but water content will not be determined during calibrations.
Record the mass difference of the sample cylinder. 14.9 Calculate the calibration factor according to Eq 3 below in the Calculation section.
TABLE 2 Recommended Parameters for Liquefied Gas Samplers
Parameters Mitsubishi Metrohm ECH
Heater Temperature (°C) 60 °C 80 °C 60 °C to 80 °C
Flow Rate (mL/min) 500 <2400 <2000
Number of Replicates 3 3 3
Procedure
Set up the coulometric KF titrator and liquefied gas sampler according to the manufacturer’s instructions and add the proper amount of reagents.
The KF cell shall be in a dry or conditioned state prior to introduction of sample. Follow the manufacturer’s instructions for drying or conditioning the electrolytic cell prior to the start of the analysis.
Select Sample Size—The amount of pressurize sample introduced into the KF titration cell is dependent upon the quantity of water in the sample being analyzed. Determine the sample size of pressurized sample to be analyzed based upon the expected concentration of water in the sample and the suggested value listed in Table 1. Other sample sizes may be used as long as the analysis meets the performance criteria of the method.
Program the liquefied gas sampler following the manufacturer’s recommendations. Table 2 outlines recommended or suggested parameter settings for three different manufacturers of liquefied gas samplers.
Connect the sample cylinder to the liquefied gas sampler taking care to ensure the connections are tight, close the sample introduction valve, and check the connections checked for leaks prior to proceeding. Position the sample cylinder so that liquid phase is delivered to the liquefied gas sampler.
Care should be taken to ensure sufficient cylinder pressure is maintained to keep the sample in the liquid phase during the analysis.
The liquefied gas sampler should be programmed for the number of analytical replicates prior to the start of the analysis.
Start the analysis following directions of the manufacturer of the liquefied gas sampler and coulometric KF titrator.
Follow the manufacturer’s procedure and protocol for sampling and purging of the liquefied gas sampler.
Purge the liquefied gas sampler with new sample from the sample cylinder before starting the first analysis. The initial purge step is important to ensure a representative sample is analyzed for moisture.
Once the analysis and KF titration is complete, the amount of water (in micrograms) measured in the sample will appear on the instrument’s display and the concentration calculated using the equations in Section 16.
To connect another sample cylinder to the liquefied gas sampler, the pressurized sample in the transfer line from the sample cylinder shall be vented by following the manufacturer’s procedure and protocol.
Once all the residual sample is vented from the liquefied gas sampler and sampling lines, disconnect the sample cylinder.
To analyze another sample, repeat steps 15.5 through 15.10 as in D7995.
Calculation
Calculate the gas calibration factor as follows:
C = V⁄ M (3)
where:
C = calibration factor, L/g,
V = volume of gas passing through the MFM, L, and
M = sample mass difference before passing through the MFM and after passing through the MFM, g. Calculate the water content of the sample as follows:
µg/g H2O = W/ V⁄ C (4)
where:
W = amount of H2O titrated, µg,
V = volume of gas passing through MFM, L, and
C = gas calibration factor.
NOTE 3—Karl Fischer titration results are usually expressed in µg/g which is numerically equivalent to mg/kg.
Report
Report the result as milligrams of water per kilograms of sample to the nearest whole unit. 17.1.1 One replicate is equal to one result. Averaging more than one replicate for one result is permitted.
Intermediate Precision and Bias
The precision of this test method is based on an intralaboratory study conducted in 2018. A single laboratory participated in this study, testing a finished liquid butane sample spiked at three concentrations. Every test result” represents an individual measurement. The laboratory reported 25 replicate test results for each concentration. Practice D6300 was followed for the design and analysis of this interim repeatability data; the details are given in ASTM Research Report No. RR:D02-1904.7.
Repeatability (r)—The difference between repetitive results obtained by the same operator in a given laboratory applying the same test method with the same apparatus under constant operating conditions on identical test material within short intervals of time would in the long run, in the normal and correct operation of the test method, exceed the following values in only in one case in 20.
Repeatability limits can be interpreted as the maximum difference between two results, obtained under repeatability conditions that are accepted as plausible due to random causes under normal and correct operation of the test method.
Repeatability standard deviations are listed in Table 3.
Reproducibility limit (R)—The difference between two single and independent results obtained by different operators applying the same test method in different laboratories using different apparatus on identical test material would, in the long run, in the normal and correct operation of the test method, exceed the following values only in one case in 20.
Reproducibility can be interpreted as the maximum difference between two results, obtained under reproducibility conditions that are accepted as plausible due to random causes under normal and correct operation of the test method.
TABLE 3 Total Water in Liquid Butane (mg/kg)
Sample Concentration (water) Average x¯ Repeatability Standard Deviation Sr
Low (1.0 mg/kg) 1.536 0.554
Middle (25 mg/kg) 26.346 0.510
High (100 mg/kg) 98.366 3.972
Reproducibility limits cannot be calculated from a single laboratory’s test results.
The above terms (repeatability limit and reproducibility limit) are used as specified in Practice E177.
Any judgment in accordance with r as defined, would normally have an approximate 95 % probability of being correct, however the precision statistics obtained in this ILS must not be treated as exact mathematical quantities that are applicable to all circumstances and uses. The limited number of laboratories reporting replicate results essentially guarantees that there will be times when differences greater than predicted by the ILS results will arise, sometimes with considerably greater or smaller frequency than the 95 % probability limit would imply. Consider the repeatability limit as a general guide, and the associated probability of 95 % as only a rough indicator of what can be expected.
Bias—At the time of the study, there was no accepted reference material suitable for determining the bias for this test method, therefore no statement on bias is being made.
The precision statement was determined through statistical examination of 75 test results, from a single laboratory, on three (3) concentrations of water-spiked liquid butane samples.
Keywords
butane; chemical analysis—petroleum products; dimethyl ether; DME; Karl Fischer; KF titration; liquefied petroleum gas; LPG; moisture; water.