Assessing Elemental Content: Purposes, Techniques, and Insights
Measuring the elemental content serves several critical purposes that are vital for ensuring the performance, reliability, and longevity of both the lubricants and fuels. Regularly checking the elemental content ensures that the lubricant maintains its specified composition, ensuring consistent performance. This is particularly important during manufacturing and before the lubricant is applied in critical machinery. Further reasons for conducting such measurements are the following:
- Detection of Wear Metals:
As equipment components wear down during operation, metallic particles are generated and circulated throughout the lubrication system. Analyzing the elemental content helps in detecting wear metals that are released into the lubricant from machinery components. This can indicate the wear rate of parts, allowing for predictive maintenance and preventing catastrophic failures. In case of machinery failure, analyzing the elemental content of the lubricant can help identify the root cause. - Contaminant Identification:
The presence of certain elements can signify contamination from sources like dirt, coolant leaks, or sea water. Identifying these contaminants helps to understand error modes and narrow down the possible causes. - Additive Depletion:
Lubricants contain various additives that enhance performance by providing properties like anti-wear, anti-oxidation, and corrosion resistance. Measuring these elements helps in assessing the condition and remaining useful life of the lubricant. Professionals in the industry typically monitor potential changes in concentration over time, starting from the initial concentration of the fresh lubricant.
Table 1 summarizes the most important elements linked to their main origin.
| Origin | Elements | Typical trend during use |
| Wear Materials | Iron, Chromium, Lead, Copper, Tin, Aluminium, Nickel |
upwards |
| Contaminants | Silicon, Sodium, Potassium | upwards |
| Additives | Boron, Phosphorus, Zinc, Calcium, Barium, Magnesium, Molybdenum |
downwards |
[Table 1.] Elements of interest for elemental analysis.
Methods to perform elemental analysis
The history of elemental analysis goes back to the 1940s and 1950s when it was used in the railroad industry to determine the presence of wear metals in diesel engine oils.
In its present form, elemental analysis is used to determine the concentrations of more than 30 different elements.
Nearly all oil analysis labs utilise one of two types of atomic emission spectrometers: either an inductively coupled plasma (ICP) instrument or a rotating disc electrode (RDE) instrument. The primary difference between these two lies in the method of sample vaporisation and atom excitation by the high-energy source. In an ICP instrument, the oil is injected into a high-temperature argon plasma, where the atoms are vaporised, excited, and subsequently emit light. In an RDE spectrometer, also known as an “Arc-Spark” instrument, the oil is vaporised and excited using a high-voltage discharge between an electrode and a rotating carbon disc.
Besides the different vaporisation and excitation methods, the rest of the instrument functions similarly in both ICP and RDE spectrometers. The emitted light from the excited atoms is collected and focused onto the spectrometer’s slit. Inside the spectrometer, a diffraction grating, which functions like a prism, splits the light into discrete wavelengths based on their angle of diffraction.
The light intensity at each angle, typically referred to as a channel, is measured using a light-sensitive photodiode. The resulting voltage signal is then converted into a concentration in parts per million (ppm) through a calibration procedure. Although ICP and RDE measure the elemental composition of a sample using a common principle, the practical implementation of the two techniques is radically different.
An ICP requires sample preparation, typically including acid pre-treatment and microwave digestion, and a supply of carrier gas. As the measurement procedure is quite involved, ICPs are typically found in well-equipped labs with skilled operators. The RDE method, on the other hand, does not require any sample preparation and the sample is measured as received. The instrumentation requires stable temperature conditions, but otherwise no additional materials. This means that the RDE method can be used not only in an industrial environment, but also in the field or at remote locations.
The ICP method offers the advantage of sub-ppm detection limits as well as the ability to detect sulphur in routine analysis. The RDE instruments typically do not detect sulphur due to the lack of a vacuum system but offer ppm detection limits for up to 32 elements, including the relevant elements found as wear in oil additive packages. For used oil analysis, RDE offers the advantage that it can detect particles up to 10 μm, whereas ICP typically only detects particles below 5 μm.
Data comparison
To investigate the relation between the elemental composition determined by ICP and RDE, 35 samples of gear oil, hydraulic oils and motor oils were collected from a steel plant. Both fresh and in-service oils were used for the study. The samples were measured using a 2500 ICP-OES spectrometer from Agilent according to ASTM D5185 and an ERAOIL RDE-OES spectrometer according to ASTM 6595. For the ICP measurements, the samples were treated with nitric acid and hydrogen peroxide and then microwave digested at 190°C. The RDE samples were measured without sample preparation.
Elements that were detected by ICP in at least 20% of the samples were used for the comparison.
In general, the ICP and RDE results match well for both the detected wear metals as well as elements that can be assigned to additives. Figure 1 shows the correlation between iron (Fe) and zinc (Zn) levels in the samples. Table 2 summarizes the obtained correlations for all investigated elements.
| Element | # Samples | Range | Corr | R² |
|---|---|---|---|---|
| B (Boron) | 23 | 0–100 | 1.02 | 0.96 |
| Ca (Calcium) | 22 | 0–70 | 1.26 | 0.95 |
| Cu (Copper) | 17 | 0–300 | 1.39 | 0.99 |
| Fe (Iron) | 29 | 0–200 | 1.20 | 0.97 |
| Mg (Magnesium) | 10 | 0–800 | 1.18 | 0.99 |
| P (Phosphorus) | 35 | 0–1200 | 0.92 | 0.96 |
| Si (Silicon) | 28 | 0–20 | 0.89 | 0.99 |
| Zn (Zinc) | 33 | 0–1000 | 0.89 | 0.99 |
[Table 2.] The resulting correlation between ICP and RDE for the 8 investigated elements.
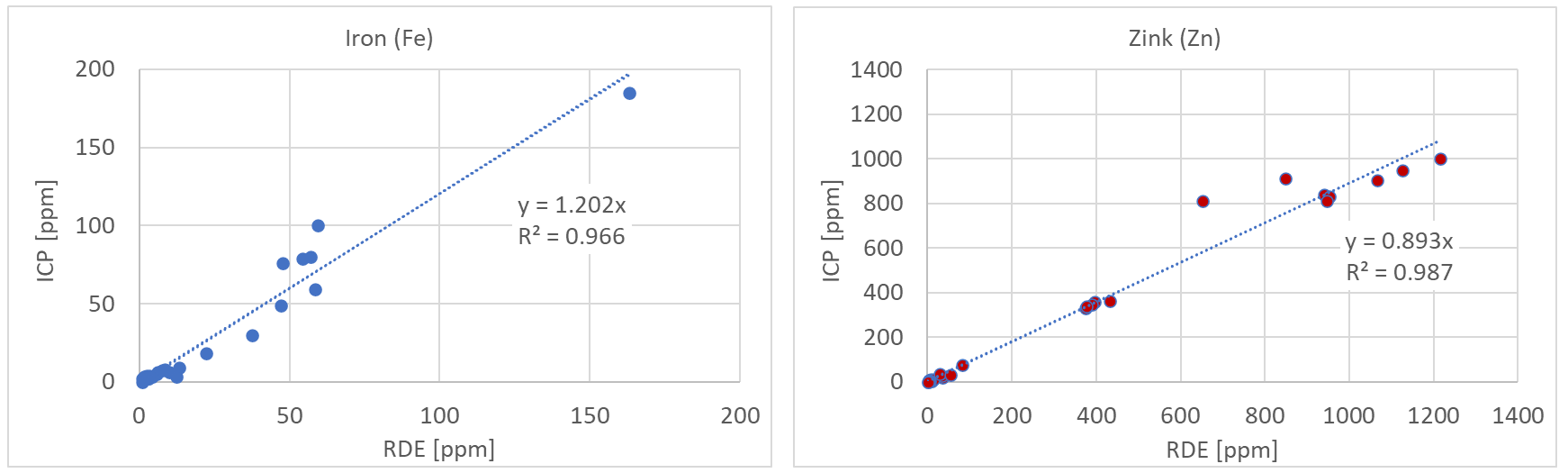
[Figure 1] Iron and zinc as determined by ICP and RDE.
All investigated elements showed a high degree of correlation between the two methods with R2 factors above 0.95 and correlation factors close to unity. The deviation of the correlation factor from 1 is probably not related to a difference in calibration, as both techniques are calibrated with similar certified reference materials. It is possible that the difference is related to the sample preparation used for ICP and the different ways the two methods excite the atoms in the sample. In this way, the difference only appears for actual samples and not for the artificial metallo-organic complexes used to calibrate the instruments.
For a sub-set of the collected samples, a clear difference in the results from the ICP and the RDE could be shown for one element. The collected motor oils (diesel engine oils) had been overbased to boost the TBN value of the oil and consequently had elevated levels of calcium. The results for calcium on these overbased oils are shown in Figure 2.
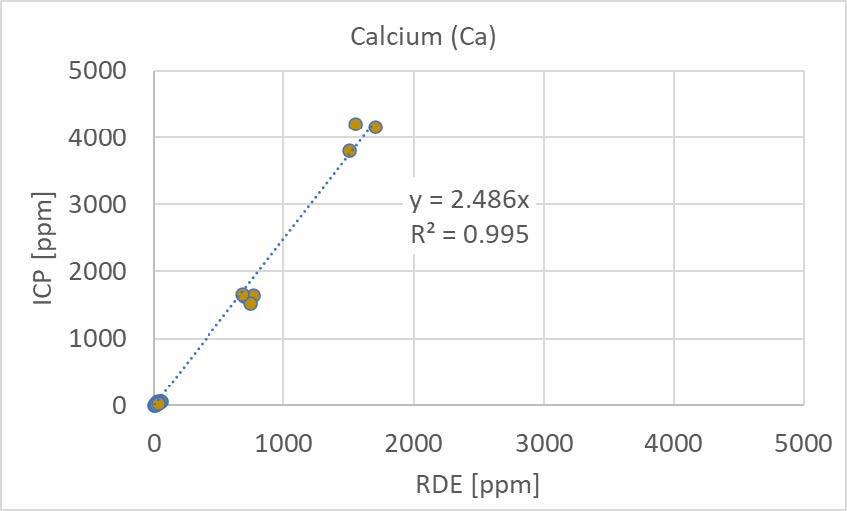
[Figure 2] Calcium in overbased oils as determined with ICP and RDE.
For the high-concentration samples, the RDE method significantly underestimates the actual concentration (correlation factor 2.5) but still shows a high degree of correlation (R2=0.99).
Figure 3 shows the repeatability of the tests and the ERAOIL results shows very high repeatability for majority of parameters.
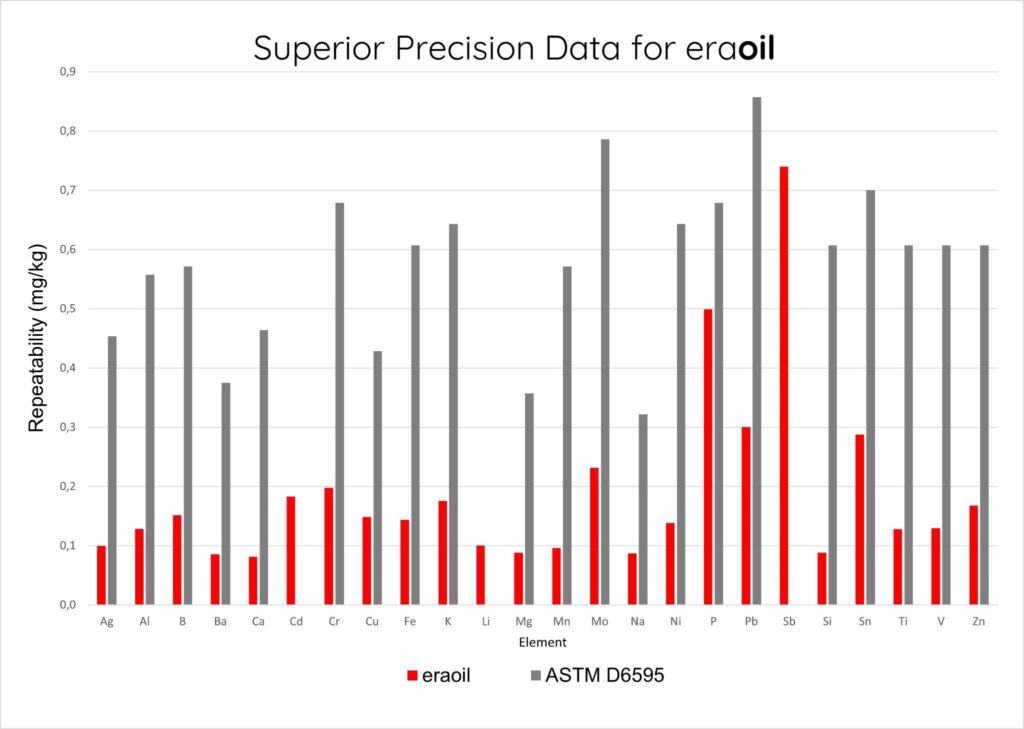
[Figure 3] High repeatability of ERAOIL results on the majority of parameters.
For the overbased oils, the results of RDE without sample preparation will underestimate the calcium concentration. As the different oils all had different additive packages, it is likely that this effect is general and applies to all types of calcium detergents used to overbase oils. However, the correlation between RDE without sample preparation and ICP is still excellent (R2=0.99), which shows that the difference in absolute values can be accounted for in the calibration procedure for overbased oils. The more cumbersome alternative is to employ the same sample preparation procedure for both methods.
Conclusion
Measuring the elemental content in all types of lubricants is essential to ensure their performance, reliability and longevity, as well as that of the machines in which they are used. Regular analysis detects wear metals, providing information on the wear rate of parts, contributing to predictive maintenance and preventing breakdowns. It also identifies contaminants and detects additive depletion that affects lubricant performance. Typically, oil analysis is performed for this purpose using either an inductively coupled plasma (ICP) or rotating disk electrode (RDE) device. ICP provides accurate results with prior sample preparation, while RDE is much easier to use and provides comparable results. A comparative study has shown that the measurement results between ICP and RDE generally correlate very well.




