ASTM D6304-16e1
Standard Test Method for Determination of Water in Petroleum Products, Lubricating Oils, and Additives by Coulometric Karl Fischer Titration
This test method covers the direct determination of water in the range of 10 mg/kg to 25 000 mg/kg entrained water in petroleum products and hydrocarbons using automated instrumentation. This test method also covers the indirect analysis of water thermally removed from samples and swept with dry inert gas into the Karl Fischer titration cell. Mercaptan, sulfide (S− or H2S), sulfur, and other compounds are known to interfere with this test method (see Section 5 in D6304).ever growing common ailments.
This test method is intended for use with commercially available coulometric Karl Fischer reagents and for the determination of water in additives, lube oils, base oils, automatic transmission fluids, hydrocarbon solvents, and other petroleum products. By proper choice of the sample size, this test method may be used for the determination of water from mg/kg to percent level concentrations.
Cannabidiol, better known as CBD, is becoming popular natural remedy used for ever growing common ailments.
The determination of moisture in a vast range of products and materials utilizing the Karl Fischer reaction. The accurate determination of water content in organic substances, nonaqueous solvents and hydrocarbons is vital to quality assessment, quality control, synthesis, handling, transportation and sales
Referenced Documents
ASTM Standards:
D1193 Specification for Reagent Water
D1298 Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method
D4052 Test Method for Density, Relative Density, or API Gravity of Liquids by Digital Density Meter
D4057 Practice for Manual Sampling of Petroleum and Petroleum Products
D4177 Practice for Automatic Sampling of Petroleum and Petroleum Products
D5854 Practice for Mixing and Handling of Liquid Samples of Petroleum and Petroleum Products
E203 Test Method for Water Using Volumetric Karl Fischer Titration
Summary of Test Method
An aliquot is injected into the titration vessel of a coulometric Karl Fischer apparatus in which iodine for the Karl Fisher reaction is generated coulometrically at the anode. When all of the water has been titrated, excess iodine is detected by an electrometric end point detector and the titration is terminated. Based on the stoichiometry of the reaction, 1 mol of iodine reacts with 1 mol of water; thus, the quantity of water is proportional to the total integrated current according to Faraday’s Law. The sample injection can be done either by mass or volume. The viscous samples can be analyzed by using a water vaporizer accessory that heats the sample in the evaporation chamber, and the vaporized water is carried into the Karl Fischer titration cell by a dry inert carrier gas.
Significance and Use
Coulometric Karl Fischer (KF) titration is a highly accurate technique employed for moisture determination in non-aqueous solvents. CBD oil is made by extracting CBD from the cannabis plant, then diluting in a carrier oil like coconut or hemp seed oil. It’s gaining momentum in the health and wellness world, with some scientific studies confirming it may ease symptoms of ailments like chronic pain and anxiety. Though research is limited at this time, CBD has been shown to ease symptoms related to epilepsy and Parkinson’s disease. CBD was also shown to reduce the progression of Alzheimer’s disease in test-tube and animal studies.

Basic chemical structure of CBD
Apparatus
Coulometric Karl Fischer Apparatus (using electrometric end point)—A number of automatic coulometric Karl Fischer titration assemblies consisting of titration cell, platinum electrodes, magnetic stirrer, and a control unit are available on the market. Instructions for operation of these devices are provided by the manufacturers and are not described herein. Figure 1,2 and 3 shows different models including head space and viscous sample accessories for solids, liquids, resins and gases applications.
Water Vaporizer Accessory—A number of automatic water vaporizer accessories are available on the market. Instructions for the operation of these devices are provided by the manufacturers and are not described herein.
Syringes—Samples are most easily added to the titration vessel by means of accurate glass or disposable plastic syringes with “luer” fittings and hypodermic needles of suitable length to dip below the surface of the anode solution in the cell when inserted through the inlet port septum. The bores of the needles used shall be kept as small as possible, but large enough to avoid problems arising from back pressure or blocking while sampling. Suggested syringe sizes are as follows:
10 µL, with a needle long enough to dip below the surface of the anode solution in the cell when inserted through the inlet port septum and graduated for readings to the nearest 0.1 µL or better. This syringe can be used to accurately inject a small quantity of water to check reagent performance as described in the Calibration Section.
As identified in Table 1(D6304), syringes of the following capacities: 250 µL accurate to the nearest 10 µL; 500 µL accurate to the nearest 10 µL; 1 mL accurate to the nearest 0.01 mL; 2 mL accurate to the nearest 0.01 mL; and 3 mL accurate to the nearest 0.01 mL. A quality gas-tight glass syringe with a TFE-fluorocarbon plunger and “luer” fitting is recommended.
Figure 1. Aquamax Pro Oil Karl Fischer Titrator

Figure 2. Bench Top and Portable Aquamax Plus Titrator
Reagents and Materials
Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society,
Purity of Water—Unless otherwise indicated, references to water shall be understood to mean reagent water as defined by Type II of Specification D1193.
Xylene— Reagent Grade, less than 100 mg ⁄kg to 200 mg ⁄kg water, dried over a molecular sieve (Warning— Flammable. Vapor harmful).
Karl Fischer Reagent— standard commercially available reagents for coulometric Karl Fischer titrations.
Anode Solution—Mix six parts of commercial Karl Fischer anode solution with four parts of reagent grade xylene on a volume basis. Newly made Karl Fischer anode solution shall be used. Other proportions of anode solution and xylene may be used and determined for a particular reagent, apparatus, and sample tested. Some samples may not require any xylene, whereas others will require the solvent effect of the xylene (Warning—Flammable, toxic if inhaled, swallowed, or absorbed through skin).
NOTE 1—Toluene may be used in place of xylene. However, the precision data in Precision and Bias Section were obtained using xylene.
Cathode Solution—Use standard commercially available cathode Karl Fischer solution. Newly made solution shall be used (Warning—Flammable, may be fatal if inhaled, swallowed, or absorbed through skin. Possible cancer hazard.).
If the sample to be analyzed contains ketone, use commercially available reagents that have been specially modified for use with ketones. NOTE 2—Some laboratories add the ketone suppressing reagent as part of their standard analytical procedure since often the laboratory does not know whether the sample contains ketone.
Hexane— Reagent Grade, less than 100 mg ⁄kg to 200 mg ⁄kg water (Warning—Flammable. Vapor harmful). Dried over molecular sieve.
White Mineral Oil—Also called paraffin oil or mineral oil. Reagent grade.
Molecular Sieve— 5Å—8 to 12 mesh.
Sampling
Sampling is defined as all the steps required to obtain an aliquot representative of the contents of any pipe, tank, or other system and to place the sample into a container for analysis by a laboratory or test facility.
Laboratory Sample—The sample of petroleum product presented to the laboratory or test facility for analysis by this test method. Only representative samples obtained as specified in Practices D4057 and D4177 and handled and mixed in accordance with Practice D5854 shall be used to obtain the laboratory sample.
NOTE 3—Examples of laboratory samples include bottles from a manual sampling, receptacles from automatic samplers, and storage containers holding a product from a previous analysis.
Test Specimen—The aliquot obtained from the laboratory sample for analysis by this test method. Once drawn, use the entire portion of the test specimen in the analysis.
Select the test specimen size as indicated in Table 1 (D6304), based on the expected water content.
Interferences
A number of substances and classes of compounds associated with condensation or oxidation-reduction reactions interferes in the determination of water by Karl Fischer titration. In petroleum products, the most common interferences are mercaptans and sulfides. At levels of less than 500 mg ⁄kg as sulfur, the interference from these compounds is insignificant for water concentrations greater than 0.02 % by mass. For more information on substances that interfere in the determination of water by the Karl Fischer titration method, see Test Method E203. Some interferences, such as ketones, may be overcome if the appropriate reagents are used.
The significance of the mercaptan and sulfide interference on the Karl Fischer titration for water in the 10 mg ⁄kg to 200 mg ⁄kg range has not been determined experimentally. At these low water concentrations, however, the interference may be expected to be significant for mercaptan and sulfide concentrations of greater than 500 mg ⁄kg as sulfur.
The Aquamax KF PRO Oil shown in Fig.1 is the perfect instrument to measure ppm water in oils and fuels without the worry of interference side reactions caused by additives or S/SH-. The unique “closed loop” concept illustrated in Fig.4 avoids methanol to evaporate from the KF reagent. Meaning no additional carrier gas necessary. Directly injecting the sample in to the oven means no blank value is required, making the Aquamax KF PRO Oil a truly accurate, trace level water in petroleum products titrator.
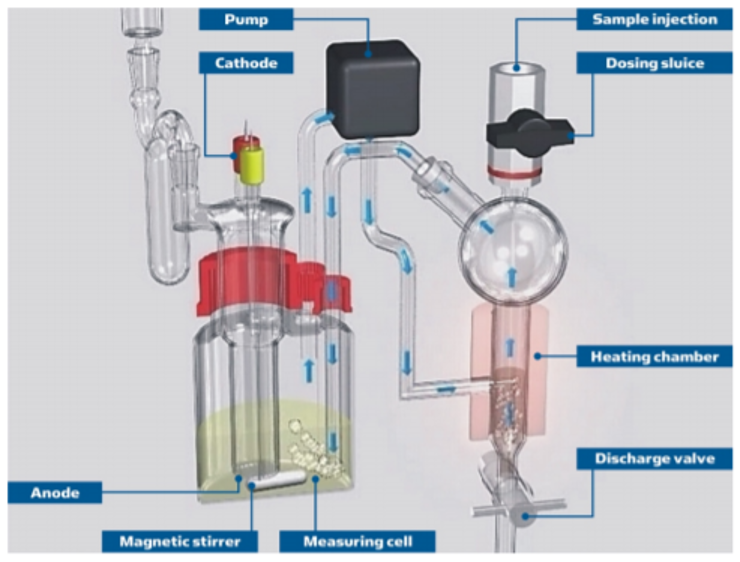
Figure 4. Closed Loop Carrier Gas design
Preparation of Apparatus
Follow the manufacturer’s directions for preparation and operation of the titration apparatus.
Seal all joints and connections to the vessel to prevent atmospheric moisture from entering the apparatus.
Add the Karl Fischer anode solution to the anode (outer) compartment. Add the solution to the level recommended by the manufacturer.
Add the Karl Fischer cathode solution to the cathode (inner) compartment. Add the solution to a level 2 mm to 3 mm below the level of the solution in the anode compartment.
Turn on the apparatus and start the magnetic stirrer for a smooth stirring action. Allow the residual moisture in the titration vessel to be titrated until the end point is reached. Do not proceed beyond this stage until the background current (or background titration rate) is constant and less than the maximum recommended by the manufacturer of the instrument.
NOTE 4—High background current for a prolonged period may be due to moisture on the inside walls of the titration vessel. Gentle shaking of the vessel (or more rigorous stirring action) will wash the inside with electrolyte. Keep the titrator on to allow stabilization to a low background current.
Calibration and Standardization Procedure
In principle, standardization is not necessary since the water titrated is a direct function of the coulombs of electricity consumed. However, reagent performance deteriorates with use and shall be regularly monitored by accurately injecting a known quantity of water (see 7.2 in D6304) that is representative of the typical range of water concentrations being determined in samples. As an example, one may accurately inject 10 000 µg or 10 µL of water to check reagent performance. Suggested intervals are initially with fresh reagent and then after every ten determinations (see 11.3 in D6304).
Test Procedure
Procedure A (by Mass)
Add newly made solvents to the anode and cathode compartments of the titration vessel and bring the solvent to end-point conditions as described in Section 9(D6304).
Add the petroleum product test specimen to the titration vessel using the following method:
Starting with a clean, dry syringe of suitable capacity (see Table 1 and Note 5), withdraw and discard to waste at least three portions of the sample. Immediately withdraw a further portion of sample, clean the needle with a paper tissue, and weigh the syringe and contents to the nearest 0.1 mg. Insert the needle through the inlet port septum, start the titration, and with the tip of the needle just below the liquid surface, inject the test specimen. Withdraw the syringe, wipe clean with a paper tissue, and reweigh the syringe to the nearest 0.1 mg. After the end point is reached, record the micrograms of water titrated.
NOTE 5—If the concentration of water in the sample is completely unknown, it is advisable to start with a small trial portion of sample to avoid excessive titration time and depletion of the reagents. Further adjustment of the aliquot size may then be made as necessary.
When the background current or titration rate returns to a stable reading at the end of the titration as discussed in 9.5(D6304), additional specimens may be added as per 11.2.1(D6304).
Replace the solutions when one of the following occurs and then repeat the preparation of the apparatus as in Section 9(D6304).
Persistently high and unstable background current.
Phase separation in the anode compartment or oil coating the electrodes.
The total oil content added to the titration vessel exceeds one quarter of the volume of solution in the anode compartment.
The solutions in the titration vessel are greater than one week old.
The instrument displays error messages that directly or indirectly suggest replacement of the electrolytes—see instrument operating manual.
The result of a 10 µL injection of water is outside 10 000 µg ± 200 µg.
Thoroughly clean the anode and cathode compartment with xylene if the vessel becomes contaminated with product. Never use acetone or similar ketones. Clogging of the frit separating the vessel compartments will cause instrument malfunction.
For products too viscous to draw into a syringe, add the sample to a clean, dry bottle and weigh the bottle and product. Quickly transfer the required amount of sample to the titration vessel by suitable means, such as with a dropper. Reweigh the bottle. Titrate the sample as in 11.2. 12(D6304).
Procedure B (by Volume)
Follow steps from Procedure A, taking sample by volume instead of mass.
NOTE 6—A volume aliquot of the product is titrated to an electrometric end point using a coulometric Karl Fischer apparatus. The steps described in Procedure A are followed except as noted. The volume injection method is applicable only when the vapor pressure and viscosity of the sample permit an accurate determination of the volume of the sample.
NOTE 7—The referee procedure for determination of water in liquid petroleum products by coulometric Karl Fischer titration is Procedure A, which uses a mass measurement of the product test specimen.
NOTE 8—The presence of gas bubbles in the syringe can be a source of uncertainly. The tendency of product to form gas bubbles is a function of product type and corresponding vapor pressure. Viscous products can prove to be difficult to measure volumetrically with a precision syringe.
Procedure C (Water Evaporator Accessory)
If using the water evaporator accessory for samples difficult to analyze by Procedure A or B due to sample viscosity, matrix interference, or extremely small concentrations of water (for example, <100 mg/kg), add 10 mL of white oil to the evaporator accessory. Bubble dry nitrogen gas at about 300 mL ⁄min through the oil. Heat the oil to the temperature suggested by the instrument manufacturer for a particular product type.
Dissolve 5 g ± 0.01 g of accurately weighed viscous sample in a 10 mL volumetric flask. Make up to volume with dried hexane. Shake the sample until it is completely dissolved in the solvent.
NOTE 10—All parts of the glass assembly must be thoroughly dry before use. The smallest amount of contamination by moisture will cause erroneous results. Perform several preliminary runs with known content standards to determine that the system is operating correctly. Water-inalcohol standards must be capped with rubber septa rather than rubber stoppers.
Inject 1 mL of dissolved sample into the evaporator assembly. Start the operating sequence. Follow steps 11.1 through 11.5 in Procedure A(D6304). After the end point is reached, record the micrograms of water titrated from the digital readout on the instrument.

Quality Control Checks
Confirm the performance of the instrument or the procedure each day it is in use by analyzing a QC sample that is representative of samples typically analyzed. Quality control frequency should be increased if a large number of samples are routinely analyzed. If the analysis is shown to be in statistical control, QC frequency may be reduced. Analysis of result(s) from these QC samples may be performed using control chart techniques4 or other statistical techniques. If the QC sample result determined causes the laboratory to be in an out of control situation, such as exceeding the laboratory’s control limits, investigate and take corrective action to bring the test back into control before proceeding. An ample supply of QC sample material shall be available for the intended period of use and shall be homogeneous and stable under the anticipated storage conditions. Prior to monitoring the measurement process, the user of the method needs to determine the average value and control limits of the QC sample. The QC sample precision shall be checked against the ASTM method precision to ensure data quality.
Calculations
Calculate the water concentration in mg/kg or µL/mL of the sample as follows:
water, mg/kg or µg/g = W1 /W2 or (1)
water, µL/mL = V1/ V2
where:
W1 = mass of water titrated, mg or µg (as appropriate),
W2 = mass of sample used, kg or g (as appropriate),
V1 = volume of water titrated, µL, and
V2 = volume of sample used, mL.
Calculate the water concentration, in mass or volume %, of the sample as follows:
water, mass % = W1/ 10 000 x W2 or (2)
volume % = V1 /10 x V2
where W1, W2, V1, and V2 are same as previously defined.
Use the following equations for calculating the water content of the sample in units of volume % from mass %, or of mass % from volume %.
water, volume % = water, mass % x [ density of sample at t/ density of water at t ] (3)
water, mass % = water, volume %/[ density of sample at t /density of water at t ] (4)
where:
t = test temperature.
Density may be measured using approved test methods such as Test Method D1298 and Test Method D4052. If the density is measured in units of g/mL and the density of water at test temperature is assumed to be 1 g/mL, Eq 5 and Eq 6 simplify to:
water, volume % = water, mass % x density of sample at t (g/mL) (5)
water, mass % = water, volume %/density of sample at t (g/mL) (6)
Precision and Bias
The precision of this test method as determined by the statistical examination of interlaboratory test results is as follows:
Repeatability—The difference between successive results obtained by the same operator with the same apparatus under constant operating conditions on identical test material would, in the long run, in the normal and correct operation of the test method, exceed the following values only in 1 case in 20.
Reproducibility—The difference between 2 single and independent results obtained by different operators working in different laboratories on identical test materials would, in the long run, exceed the following values in only 1 case in 20.
Volumetric Injection Mass Injection
Repeatability 0.08852 x 0.7 volume % 0.03813 x 0.6 mass %
Reproducibility 0.5248 x 0.7 volume % 0.4243 x 0.6 mass %
where x is the mean of duplicate measurements.
Bias—This test method has no bias since the coulometric determination can be defined only in terms of this test method.
Keywords
Karl Fischer titration, Coulometric titration, water




