Oil Oxidation
Rancid Ravaging of Lubricant Systems
Introduction
An in-service lubricant encounters a myriad of factors that can cause or catalyze chemical changes in the oil. This alteration of the oil’s chemical makeup subsequently affects the lubricant’s ability to do its job.
Oxidation is an inevitable yet undesirable series of chemical reactions that causes an oil’s quality and value to degrade over time. Lubricants lack immortality primarily due to the oxidative process which leads to the reduction of the oil’s ability to properly protect against friction. The oxidation process plays a major role in the alteration of the lubricant’s chemistry and ultimately results in impaired physical and chemical properties of the base oil.
This process causes the viscosity to increase and thicken largely due to sludge formation, buildup of acids that catalyze corrosion and decrease demulsibility, and formation of deposits that can block filters or stick to valves, all of which lead to the lubricant becoming unusable.
Oxidation Chemistry
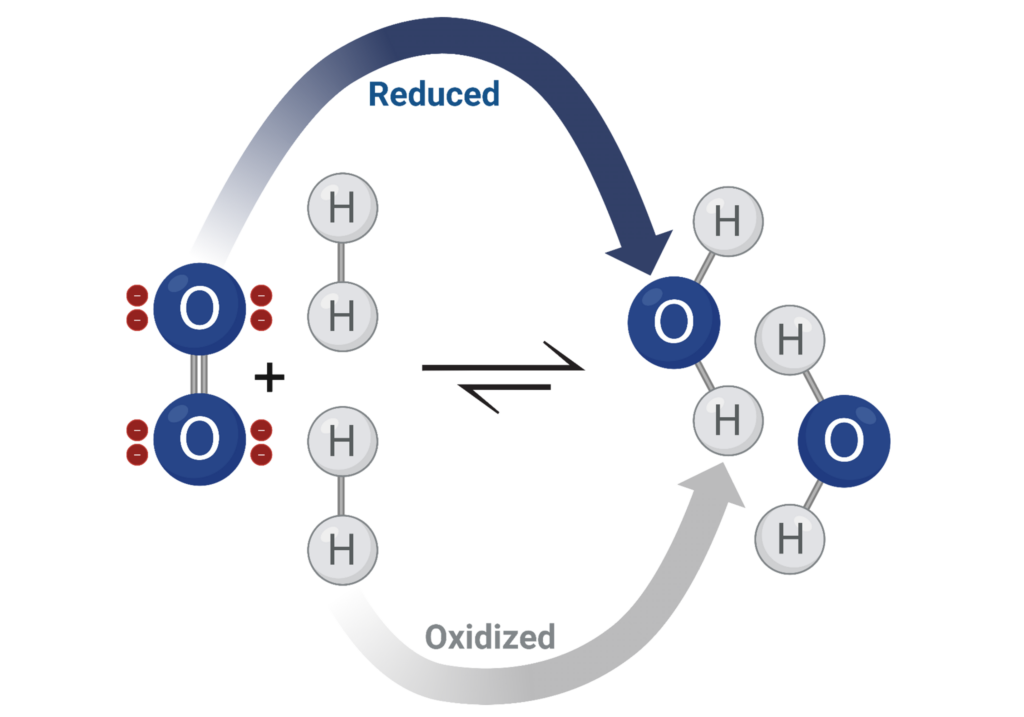
On its surface, oxidation can be defined chemically as the addition of an electronegative atom, commonly oxygen, to a compound. Oxidation is paired with a reduction process of an inverse definition: the loss of oxygen. These two half reactions occur simultaneously and that is where RedOx chemistry originates.
Oxidizing agents transfer an electronegative atom to another species in a chemical reaction. The transferred atom is most commonly oxygen because it is abundant in nature and has a high electronegativity. Electronegativity describes the chemical capability of an atom to attract electrons to fill out their valence electron shell. It is mostly affected by the amount of- and distance from- the nuclei its valence electrons exist. The oxidation number assigned to an atom is the amount of electrons that are lost or gained by that atom. This representative state is related to electronegativity and describes an atom’s oxidizing power. The sum of oxidation numbers in a neutral compound is zero. Oxygen with the second highest electronegativity on the periodic table has an oxidation number of negative two, indicating its strong oxidizing power.
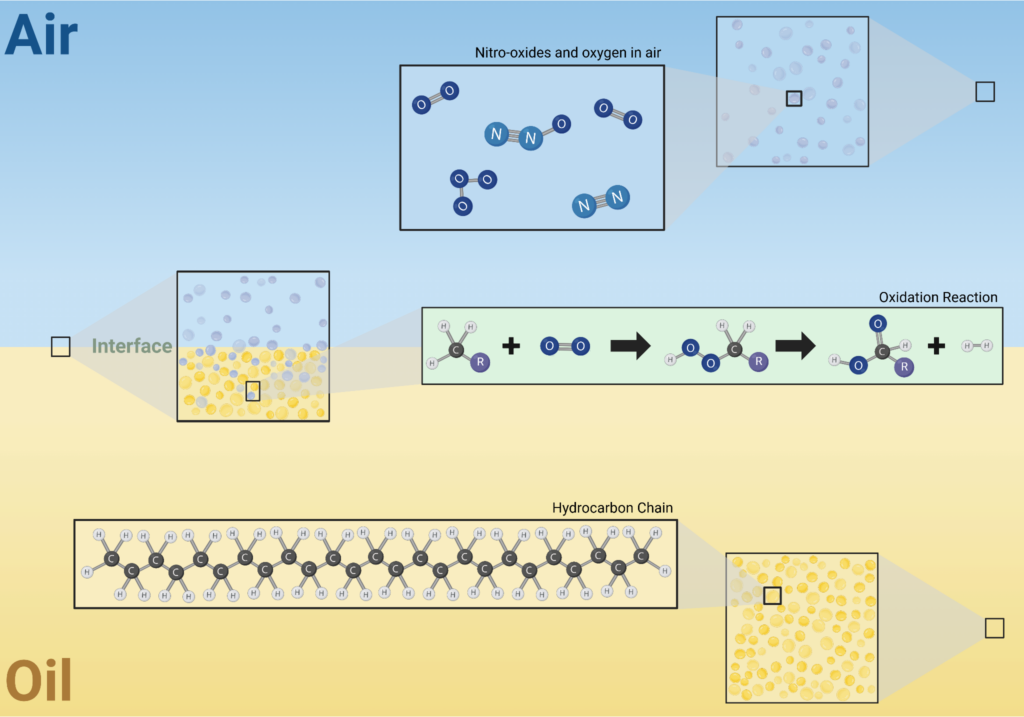
Oxidation of oil usually occurs due to the presence of oxygen found in the air. The air reacts with the hydrocarbons found in the base oil of the lubricant to ultimately produce undesirable products like acids and polymers. The hydrocarbon-based lubricant is oxidized to form the acids and polymers. Carboxylic acids are an example of a byproduct that, if allowed to congregate, will disrupt machinery and cause severe corrosion of the systems. The formation of heavy molecular weight polymers is another example of an oil-oxidized product that leads to insoluble sludge, which can clog filters and valve systems.
Steps of Oxidation
Destructive oxidation is a cyclical, free-radical-driven process. If the cycle is left uninhibited, it will continually oxidize until the oil is utterly unusable.
This cycle consists of three major stages: initiation, propagation, and termination.
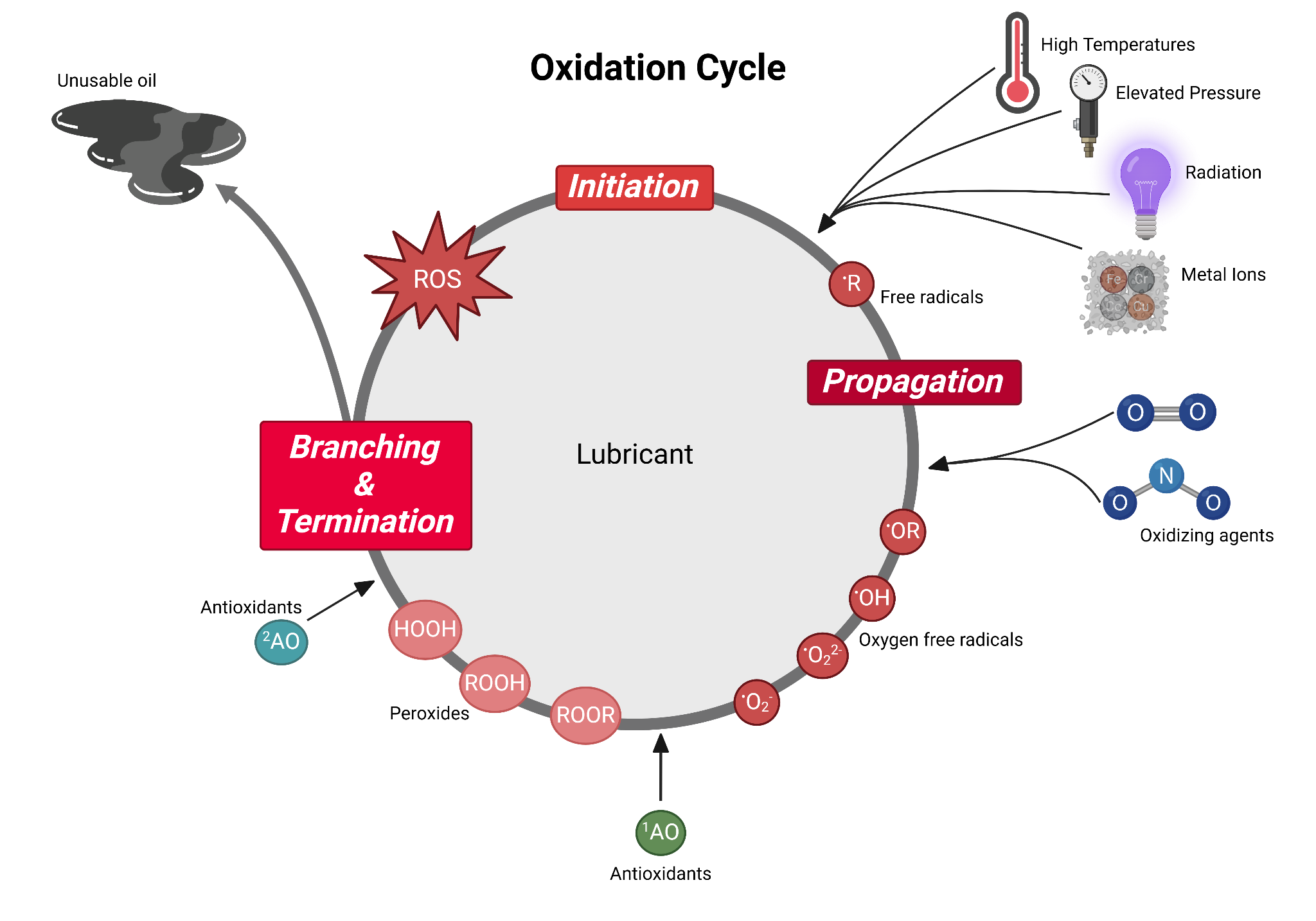
Initiation
The initiation phase revolves around the production of free radicals. These are highly reactive molecules due to their unpaired electrons which induce chemical instability. They aggressively seek out another molecule to react with in order to stabilize their own valence electron shell.
Free radicals by definition are reactive molecular species that contain at least one unpaired electron in their atomic nucleus shells and are able to exist independently. This first stage entails the mechanisms by which these free radicals are formed.
The lubricant composed of a base oil and additives undergoes some sort of reaction that initializes the oxidation process. The weaker areas in hydrocarbons react with some kind of oxidizer that forms these free radicals. The majority of refined hydrocarbon oils are mostly saturated, but persisting carbon-carbon double bonds become the targets for oxidizers. This is because strong oxidizers can more easily access the electrons in unsaturated sections making these double bonds more susceptible to attack from electrophilic molecules.
Examples of these reactants and catalysts include:
- Elemental Oxygen
Oxygen is the most notable reactant because it is the titular oxidizing agent. Atomic oxygen is critical in radical reactions at every stage so the oxygen found in the air as diatomic oxygen or ozone, or even the oxygen within water or other molecules can catalyze the oxidation sequence. - Nitro-oxides
Nitrous-, nitric-, and nitrogen-dioxide are also notable prooxidants similar to oxygen. These oxidizing agents share similar oxidizing power but have lower electronegativities than elemental forms like diatomic oxygen and ozone.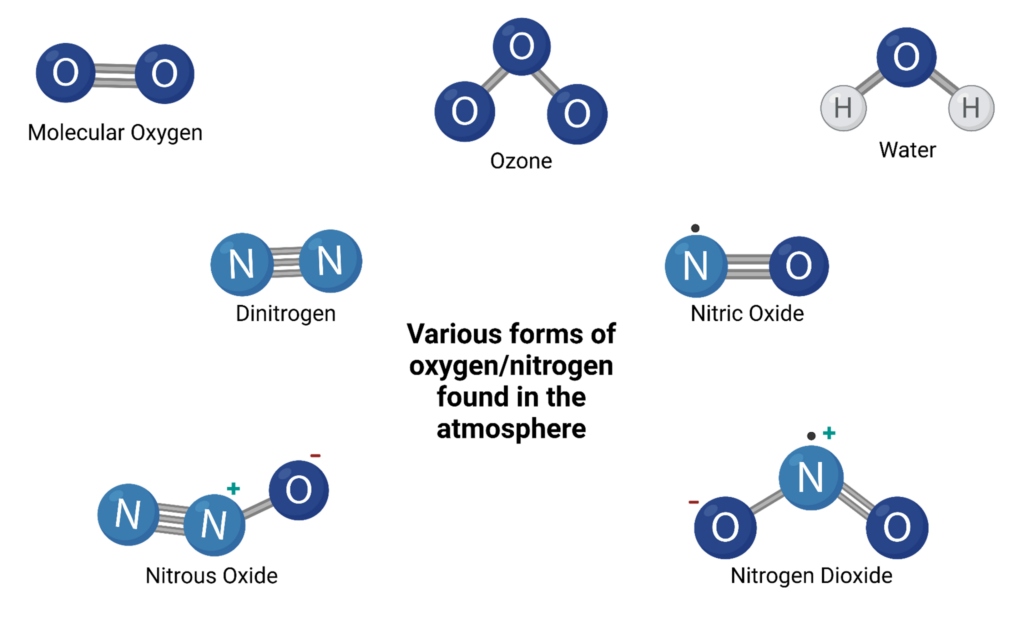
- High Temperatures
The temperature also plays a critical role in catalysis. Like most reactions, elevated temperature promotes catalytic activity. Temperature also exponentially increases oxidation rates and studies have shown that the rate of oxidation can double for every 10°C increase in operation temperature above 82°C depending on which type of oil is used. Paired with other catalysts, the effects can be multiplied.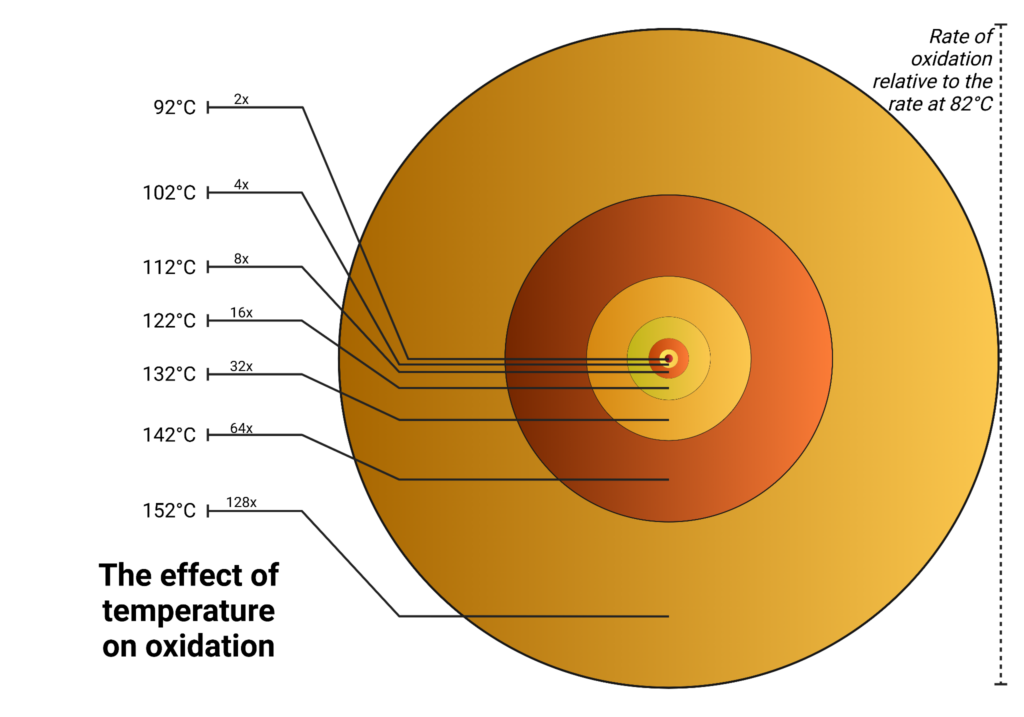
- Shear Stress
Pressure is also a common factor in catalyzing chemical reactions. This happens because of the kinetic conversion from mechanical energy into thermal energy. This is a direct result of friction; the very thing the lubricant is working to diminish. - UV Light
High energy radiation from sources like sunlight initiates the formation of free radicals because it induces the formation of more unstable molecules via the excitement of electrons. This instability allows the chemical bonds to break more easily because of the pre-excited state the electrons are already in.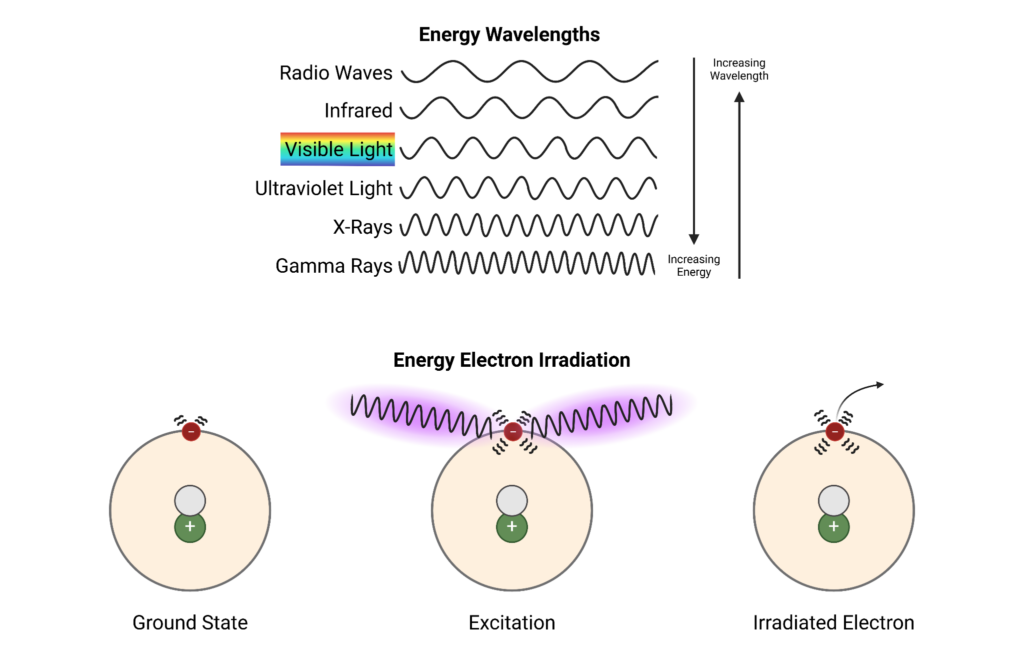
- Wear Metals
Ions found in transition metals like iron, chromium, copper, and cobalt can also accelerate oxidation because they too can act as oxidizing agents and interact in other ways with oil chemistry. Oxidation not only can create wear debris, but these worn metals can cycle back and promote further initiation.
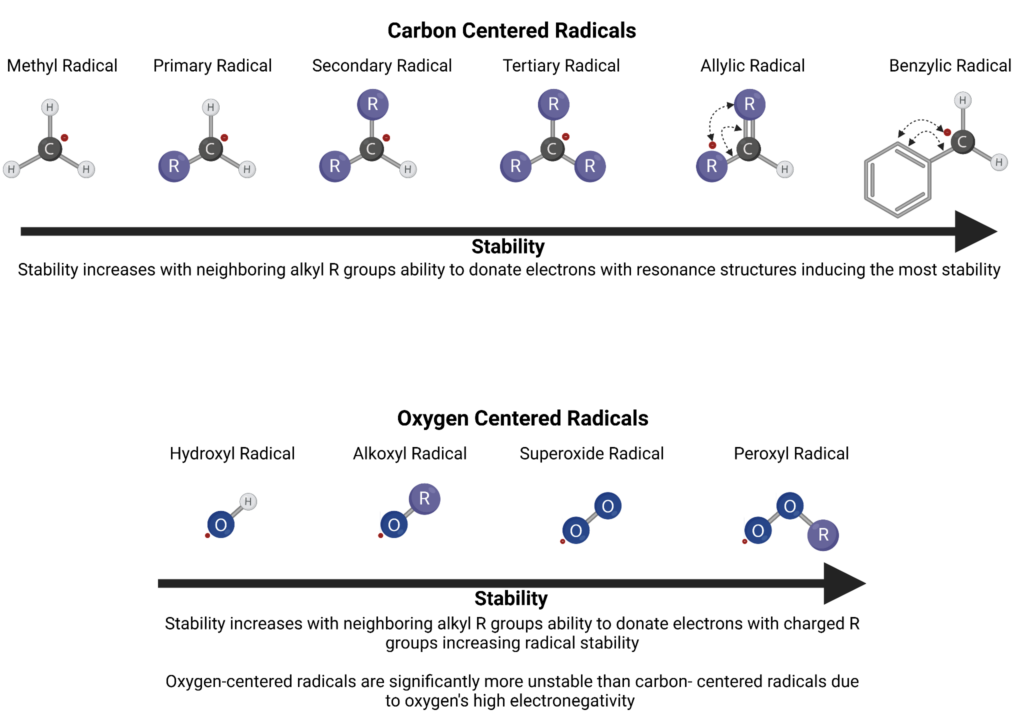
A chain initiation step induced by or incorporating these reactants and catalysts involves dehydrogenating an alkane molecule and breaking a carbon-to-carbon bond forming alkyl radicals (•R). The stability of the radicals themselves is directly determined by the strength of the carbon-to-hydrogen bond in which it was derived. Tertiary hydrogens and alpha hydrogens in aromatic hydrocarbons are the most vulnerable to oxidation. This means the arrangement of radical stability is benzylic, which is more stable than allylic, then tertiary, secondary, and primary, with phenylic radicals being the least stable form.
Even whilst the free radicals progress to the second stage, further initiations of the oxidation sequence may continue.
Propagation
Due to the unstable and reactive nature of free radicals as a result of the unpaired electrons, once they form, they immediately react again and again. This propagation phase further establishes the presence of free radicals. The original free radicals propagate the process by reacting with more hydrocarbons and free or dissolved oxygen to form more free radicals and oxygenated compounds.
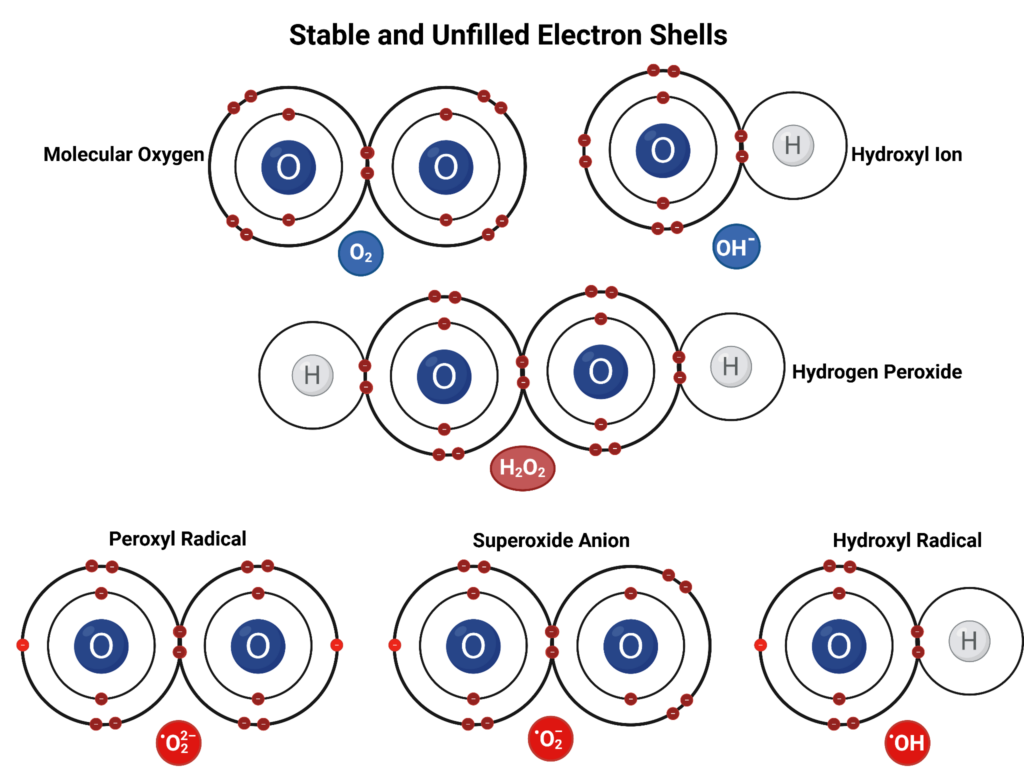
Oxygenated products like aldehydes, alcohols, ketones, and water form as a byproduct of several chain reactions of radicals and hydrocarbons. Alkoxyl and hydroxyl radicals are a species of free radicals in which they are highly reactive structural frameworks with localized radicals on the oxygen atom. The oxygen radical is bound to an alkyl group or hydrogen respectively and these radicals are nonselective, electrophilic, and oxygen-centered. Alkoxyl and hydroxyl radicals have a high bond dissociation free energy and alkoxyl radicals have weaker aliphatic C–H bonds which makes them highly oxidizing. Peroxyl radicals are more stable and occur when molecular oxygen reacts with carbon-centered radicals. Both are radical reactive oxygen species (ROS) and play major roles in oil oxidation.
Free Radical Mechanisms
Alkoxyl, hydroxyl, and peroxyl radicals are nonselective oxidizers and some of the most notable reactive oxygen species (ROS). These radicals can be formed by a direct reaction of oxygen with alkyl radicals (•R), the decomposition of alkyl peroxides (ROOH) into peroxyl (•OOR), alkoxyl (•OR), and/or hydroxyl (•OH) radicals, and even irradiation by UV light or the presence of transition metal ions can cause hemolysis of peroxides to produce these radicals.
Once formed, the alkyl radicals react quickly with a prooxidant and form peroxyl radicals. Once the peroxyl radical is formed, it abstracts a hydrogen atom from a hydrocarbon molecule and propagates the branching mechanism by generating another alkyl radical and hydroperoxide (HOOR). Peroxides are common examples of non-radical reactive oxygen species (ROS). This hydroperoxide, under suitable catalytic conditions as mentioned above, especially elevated temperatures, generates hydroxyl and alkoxyl radicals. These oxygen-containing radicals are then able to react with another hydrocarbon molecule to generate another alkyl radical and water (H2O) or alcohols (ROH).
These radicals can be produced via a variety of specific mechanisms:
- Hydrogen Abstraction
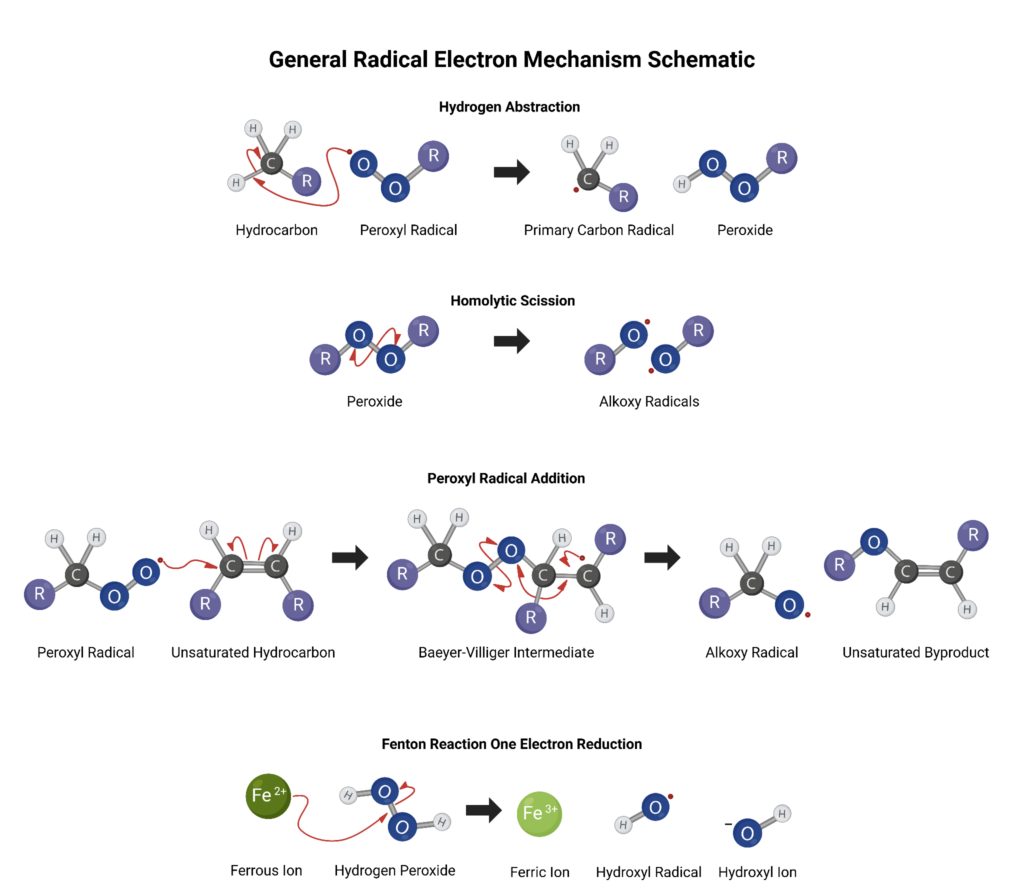
The hydrogen atom transfer (HAT) reaction is straightforward and occurs when a hydrogen atom is abstracted from a substrate like a hydrocarbon chain. The abstractor is often a free radical itself like peroxyl and produces alkoxyl radicals and this reaction is a critical component of oxidative propagation. - Homolytic Scission
Alkoxyl radicals are also the primary homolytic scission products of organic peroxides, nitrates, nitrites, hyponitrites, and hypohalites. Homolytic scission, also known as homolytic fission, hemolysis, or radical fission, occurs when a molecular bond is split and each half retains one of the original bonded electrons producing two free radicals. An example of this is when peroxides are split into alkoxyl radicals. - Peroxyl Dimerization
The dimerization of two peroxyl radicals produces alkoxyl radicals and peroxides (ROOR). With four oxygen atoms, only two can stabilize into a peroxide with the other two still unstable as radicals.
These peroxyl radicals also participate in the peroxyl radical addition (PRA) reaction. The PRA reaction occurs when a peroxyl radical adds to a carbon-to-carbon double bond and subsequently can form more free radicals via peroxide bond scission (releases an alkoxyl radical) and simply reacts again with molecular oxygen (forms another peroxyl radical). - One-Electron Reduction (OER)
A process that involves the transfer of one or two electrons from a donor to an organic substrate. An electron transfer in OER can occur from a variety of donors like neutral organic substrates and electron-rich olefins, but to form alkoxyl radicals, a transition metal commonly acts as the donor. An OER of O2 produces a superoxide anion radical (O2•–) which can undergo another OER paired with protons (H+) or two HAT reactions to form hydrogen peroxide, and another OER with a proton/HAT reaction can form a hydroxyl radical. - Fenton Reaction
This is a metal-catalyzed one electron reduction reaction in which a ferrous ion (Fe2+) is oxidized by hydrogen peroxide (H2O2) to form a ferric ion (Fe3+), hydroxide ion (OH–), and hydroxyl free radical.
In the presence of another hydrogen peroxide molecule (H2O2), the ferrous ion (Fe2+) is regenerated alongside a hydroperoxyl free radical (•OOH) and a proton, and the free radical production cycle is propagated.
In many of these cases, the product of one radical-forming reaction is a substrate of another reaction thus propagating the radical formation process in oxidation.
Termination
Oxidation can proceed either unfavorably or favorably. Oxidation proceeds unfavorably when the oxygenated compounds continue to react with the hydrocarbons and oxygen. Oxidation may also terminate favorably when the free radicals are stabilized. This termination usually occurs via chain-breaking antioxidants which effectively halt propagation by reacting with and stabilizing the radicals produced previously into inert byproducts.
Polycondensation and Polymerization
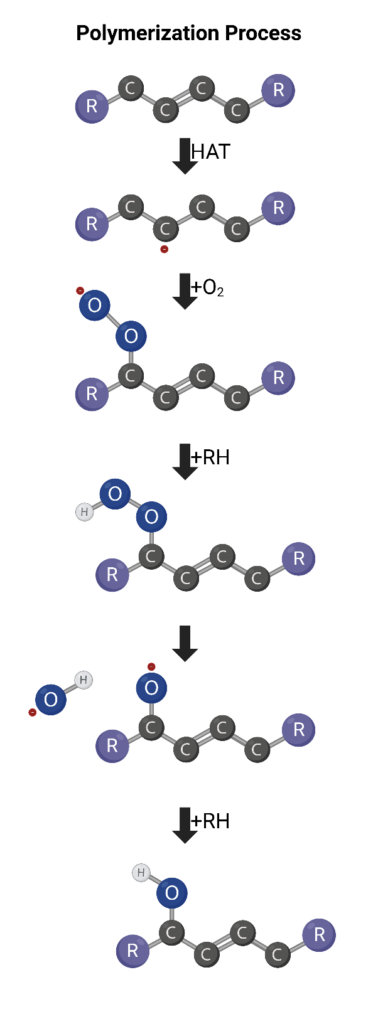 The unfavorable procession of oil oxidation occurs when oxygenated compounds like ketones continue reacting to form organic acids and heavy polymer products. High temperatures drastically increase this procession. This unfavorable termination occurs largely through polymerization and polycondensation processes.
The unfavorable procession of oil oxidation occurs when oxygenated compounds like ketones continue reacting to form organic acids and heavy polymer products. High temperatures drastically increase this procession. This unfavorable termination occurs largely through polymerization and polycondensation processes.
Aldehydes and other oxygenated compounds are oxidized further to form things like esters and carboxylic acids. Polymerization occurs when monomeric units link in addition reactions to form larger and larger products without the release of a smaller molecule. Polycondensation also results in a heavy polymer chain but reaches this product via the reaction of monomeric units’ functional groups in condensation reactions.
This increase in the molecular weight of compounds leads directly to the production of insoluble sludge and varnish deposits. Besides the formation of insoluble products, oxidized oil products also produce organic acids, and these alongside water corrosively attack the surfaces of the oil container.
The ultimate result of oxidation can be summarized to lead to the production of deposits, varnish, and sludge as well as increased oil viscosity on top of system corrosion by acids and water. These processes completely alter the original integrity of the oil producing a product entirely deficient in the characteristics that the lubricant was originally intended for.
Antioxidants
However, lubricants are designed with oxidation in mind and manufacturers incorporate antioxidants as a strategic first line of defense. Antioxidants (AOs) are additives that prolong the life of base oils by increasing their resistance to oxidation and improving thermal stability. Mechanical stress, heat, and gasses catalyze hydrocarbon molecules found in the lubricants to break down and form radicals that react with oxygen. Still, antioxidants help eliminate these radicals and prevent this breakdown.
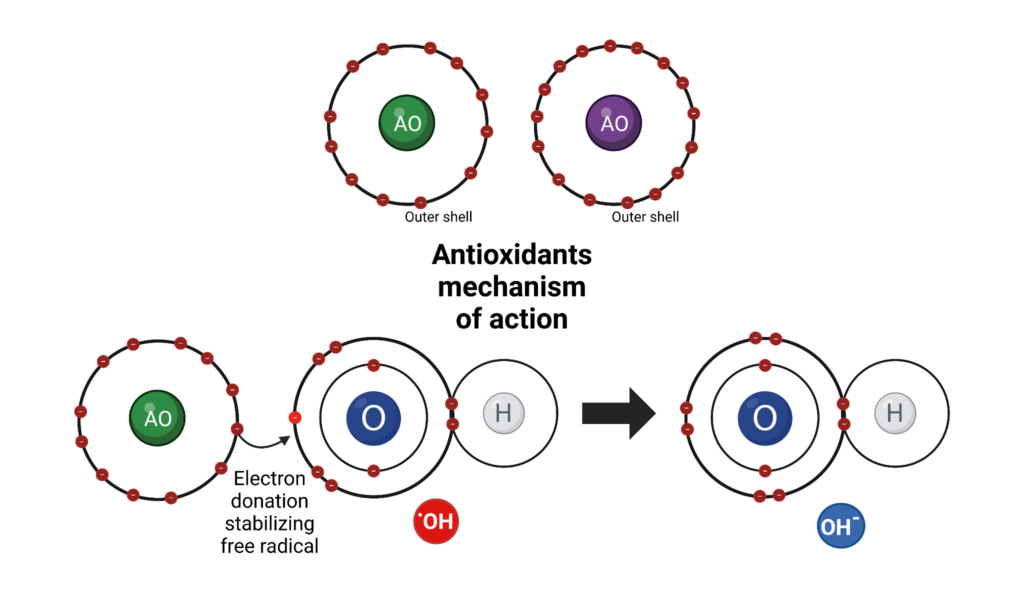
Antioxidants work as a sacrificial unit that will readily oxidize before the components of the base oil and it is the only substantial protection warding off premature failure of the lubricant. They are organic compounds typically containing nitrogen, sulfur, phosphorus, and even metals. The inert byproducts produced by antioxidants to terminate oxidation favorably can take the form of things like water and alcohols.
Some common antioxidants include sterically hindered phenols, semicarbazide and thiosemicarbazide, and phenolic or aromatic amines.
Two major types of antioxidants exist: primary and secondary AOs.
- Primary AOs
Primary AOs act as scavengers seeking out free radicals. They react quickly and stabilize radicals formed in the first two phases of oxidation; this drastically slows the degradation of the oil. These types of AOs break the chain of oxidation by donating hydrogen to free radicals which generate a resonance-stabilized compound. The donation of a labile hydrogen produces a stable hydroperoxide and these AOs form byproducts like water and alcohols.Sterically hindered phenols and some arylamines are typical types of primary AOs.
- SecondaryAOs
Secondary AOs act as hydroperoxide decomposers produced by the primary AOs. These AOs react with peroxides to inhibit the cycle and branching effects of propagation. They react with and convert peroxides into nonreactive, stable nonradicals.Phosphites and sulfur-containing compounds like thioesters and thioethers are examples of secondary AOs.
Metal deactivators are also important in deactivating metal ions mechanized by oxidation. They operate by binding to metal ions that propagate the oxidation reactions, thus preventing these metal ion-catalyzed oxidation reactions.
Limitations of AOs
Although antioxidants are necessary for lubricant formulation, it is important to note that they do not stop oxidation, but rather delay it. They also have limitations that determine the extent in which they can effectively delay oil oxidation.
Primary AOs function best at temperatures below 93°C, but lose efficacy at higher temperatures. At lower temperatures, metal deactivators cannot work efficiently, but function better at higher temperatures when metal catalysis becomes more prominent. Phenols are also known to deplete early in the lubricant’s lifetime. Amines are known to be slow to start preventative maintenance, but last longer than phenolic AOs.
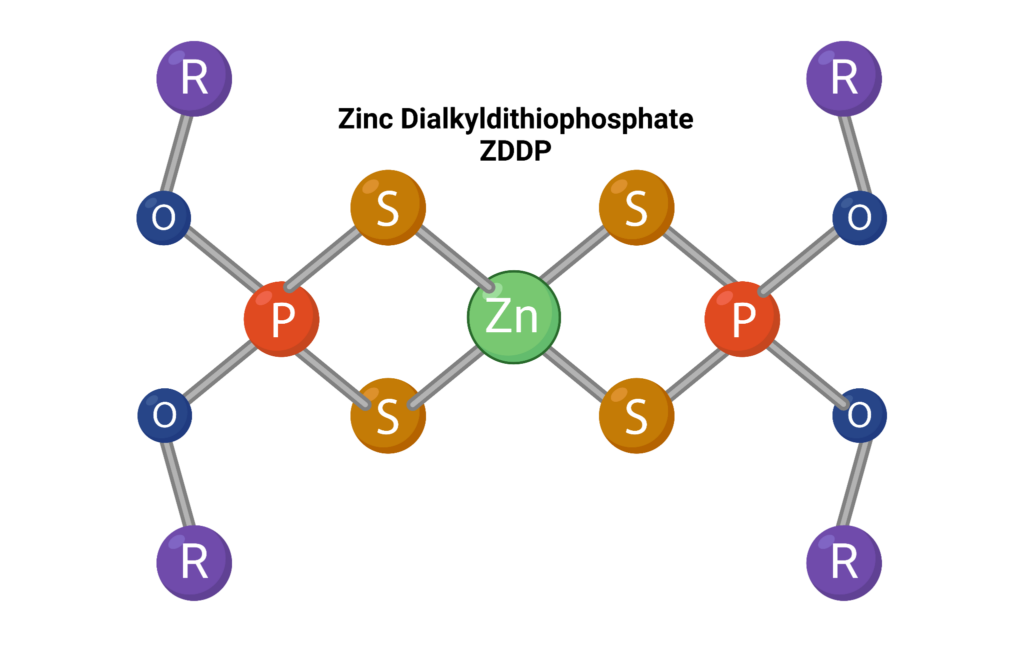
Antioxidants can also interact with other additives like anti-wear additives in the lubricant formulation. They particularly interact negatively with additives containing heavy metals. For example, zinc dialkyldithiophosphate (ZDDP) is one such additive that is affected by AOs. The AOs interact with the phosphate moieties which lessens the adherence ability of ZDDP and ultimately decreases its anti-wear effectiveness. These types of inverse interactions become more important at elevated temperatures and pressures where anti-wear capabilities are even more important.
The actual lifetime of a lubricant is entirely dependent on the lifetime of effective antioxidants. Once AOs are depleted past a point of effectiveness, the lubricant drastically loses its lubricity. The timeline for AO depletion and thus lubricant lifetime varies largely due to the factors that can speed up or slow down the usefulness of AOs like temperature, pressure, and contaminants.
Considering the limitations of certain antioxidants under specific conditions, finding the right balance of additives is incredibly important in lubricant formulation. Formulations are developed with these limitations and interactions in mind, masking each additive type’s weaknesses and maximizing their effectiveness.
Lubricant Composition Monitoring
A lubricant’s expected versus actual service life drastically differs, largely due to the catalysts and frequency in which the oil operates. Properly and regularly analyzing the chemical and physical makeup of the oil is essential to accurately determining the state of the lubricant.
There are a plethora of base oil formulations with an even wider range of additives that can be combined to make the lubricant. Understanding the composition of the lubricant when choosing which formulation to use is essential before, during, and even after use. Ensuring the lubricant is designed for the operations-specific variables in which it will be used is critical. Using a lubricant not designed for the job at hand will usually result in expedited oxidation amongst a variety of other issues, like increased acidity which leads to corrosion and decreased overall lubricity.
A variety of tests are used to qualify and quantify the decomposition of lubricant.
Color
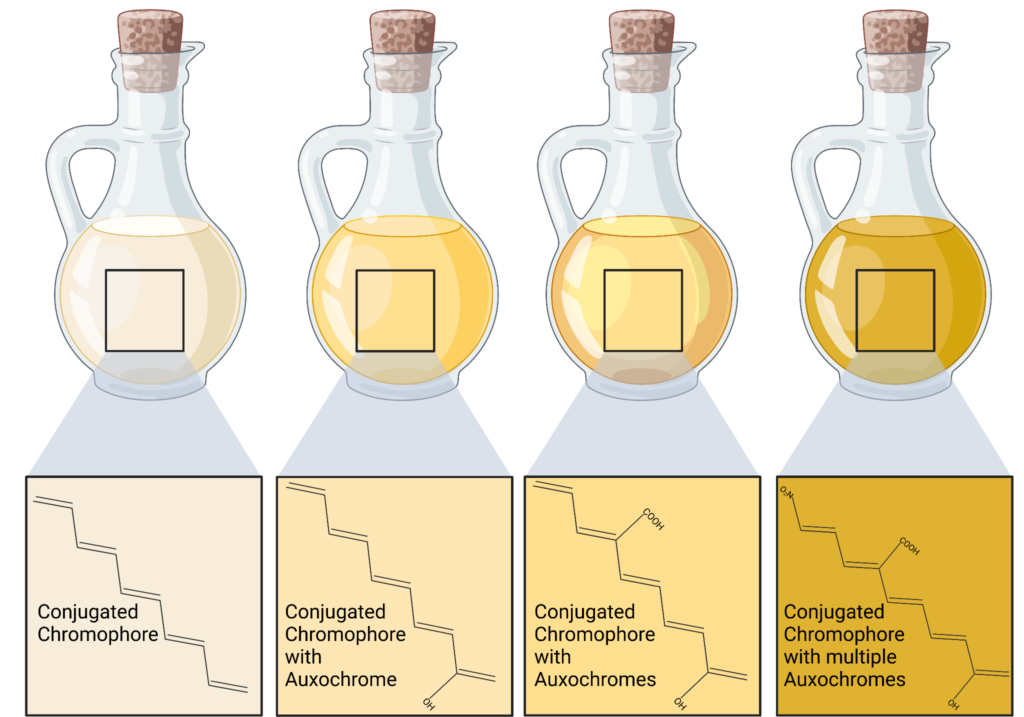
The easiest, but least reliable analysis technique is to simply look at the oil’s color. Oils will darken as they are in use due to heat, contaminants, and oxidation. This is due to the generation of chromophores and auxochromes.
Chromophores are molecules or moieties within the chemical structure that absorb particular wavelengths of visible light (400-700 nm) in the electromagnetic spectrum. For color to exist (to humans) an object has to reflect wavelengths of light within the visible light spectrum. This is only possible when a molecule absorbs visible light, has at least one chromophore, and has a conjugated system. A conjugated system is a chemical structure of alternating single and double bonds that allow for the delocalization of electrons – necessary for light absorption through the allotment of charged lattice distortions. Due to the structure of oils typically being long chains of hydrocarbons with double bonds strewn within the structure, most oils have active chromophores, giving them the yellowish hue in which they are often associated.
An auxochrome is a functional group that attaches to a chromophore which enhances the chromophore’s ability to absorb light at certain wavelengths. This supplement intensifies the color production often by extending the conjugated system via electron donation. Examples of common auxochromes include hydroxyl, amino, and carboxyl groups. As mentioned above, hydroxyl and carboxyl groups are ubiquitous oxidation products, and as more of these auxochromes are generated, the light yellowish tint intensifies into caramels and inevitably into dark browns. For example, when mechanics check the oil on a car’s dipstick, the color gives them insight into the health of the lubricant.
There are also analytical techniques that are much more accurate and comprehensive than simple qualification by eyesight. Because oxidation is generally a slow reaction accelerated primarily by heat and produces undesirable byproducts, a common approach is to measure the byproducts to analyze the stage of health of the lubricant. A classic way oxidation of an oil can be tracked is by measuring the amount of weak acids present in the oil over time. Both free radicals and the long-chain polymers produced as end-products are acidic so this test is reliable as long as a baseline value is available. This is known as the Total Acid Number (TAN) and the TAN will increase as oxidation progresses. There are a few ways to determine TAN.
Titration
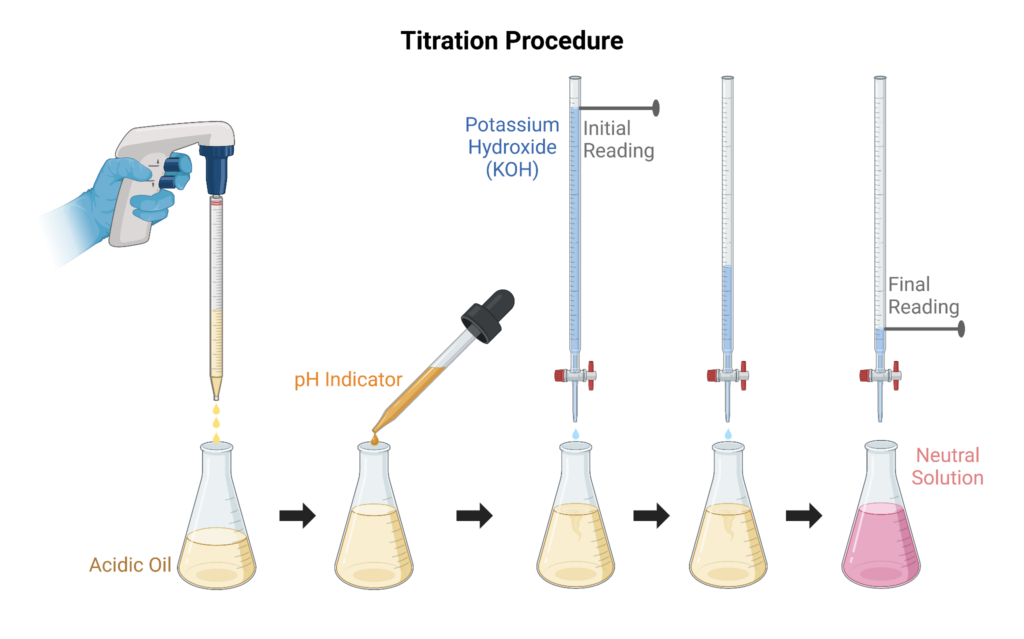
Titration operates by mechanizing the pH scale (0-14). The low end of the scale indicates a sample is highly acidic whereas the upper limit indicates greater alkalinity, and a value of 7 describes neutrality. As acidic byproducts accumulate due to the oxidation cycle, the pH will slowly become more acidic. Tracking this descent of the pH value over time provides information on the extent to which a lubricant has been oxidized. However, it is important to note, that acids can form from other pathways and may also preexist depending on the oil formulation.
To perform a titration, a weighted amount of oil is mixed with a titration solution and is then titrated with alcoholic potassium hydroxide (KOH) to determine the acid number. This value is determined by adding a known concentration of KOH, which is a strong base to the oil until the acid is neutralized. The amount of base used to neutralize the acid is then used to calculate the TAN value.
A similar approach using a strong acid like hydrochloric acid (HCL) titrated into an oil sample can help determine the Total Base Number (TBN) which can be useful for determining the concentration of components able to neutralize acidic byproducts thus extending the lubricant’s life.
Spectroscopy is another analytical tool to measure TAN and oxidative progression. It is the study of how matter interacts with radiated energy and spectroscopic analyses are used in a wide variety of industries. Infrared (IR) spectroscopy passes infrared light (780 nm – 1 mm) through a sample to determine the chemical composition. The IR light provides enough energy to vibrate the bonds within a sample and use it to determine the chemical structure. IR light is used because it has a lower energy level than visible light, allowing it to excite molecular vibrations without causing an electron to transition from one energy level to another. This makes it ideal for determining functional groups by analyzing their unique vibrational frequencies. For example, the resulting spectrum of a molecule with several different types of bonds like alkanes (CnH2n+2) and carbonyls (C=O), will depict at least two different absorption bands. The quantifiable absorption denoting the intensity of the peak is described by the Beer-Lambert law which states the IR absorbed is proportional to the concentration of the absorption species and the distance the IR light has to travel. Thus these vibrational frequencies appear as distinct peaks with measurable intensity on an IR spectrum which allows for the identification and quantification of these molecules. There are two main spectroscopic techniques used to analyze oil oxidation of lubricants.
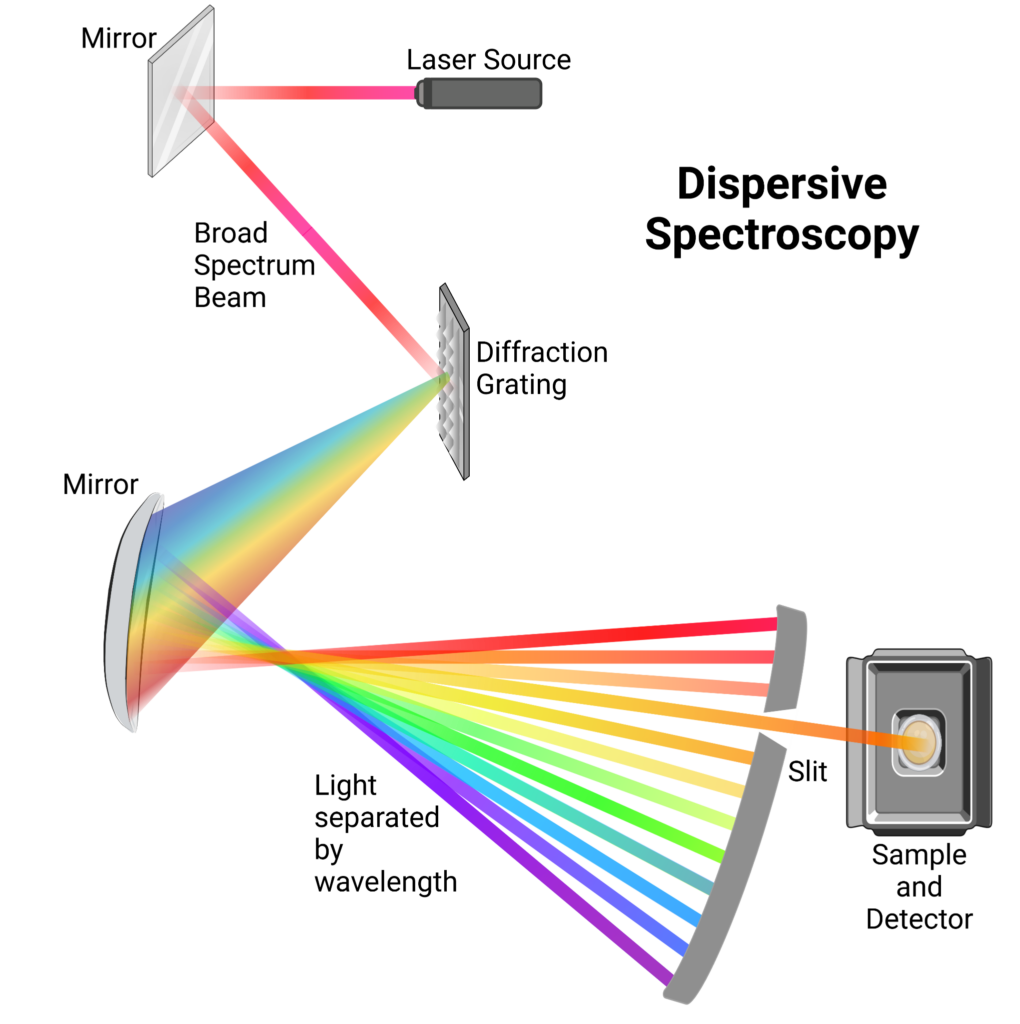
Dispersive Spectroscopy
Dispersive IR spectroscopy is the more traditional method used to measure samples. In this type of analysis, an IR light is shone onto a diffraction grating, which separates the light by its respective wavelength. The angle of the light is directly related to the wavelength of the light as it exits the grating. Once the IR beam is separated by wavelength, a slit is used to isolate a singular wavelength of IR light. The monochromatic beam is then directed to the sample and the absorbance is measured. The diffraction grating angle is then altered to isolate a different wavelength and the absorbance of that wavelength is measured. This process is repeated for every wavelength. The resulting data is then plotted to produce an IR spectrum.
Interferometric Spectroscopy
A newer and faster method, called Fourier-Transform IR spectroscopy (FT-IR) uses a different approach to produce an IR spectrum. In this method, an interferometer is used to induce IR interference with itself. Light of any wavelength travels in sinusoidal waves with peaks and troughs. The location of these peaks and troughs describes the phase of the wave. Waves with different phases have what is called a phase difference. This phase difference dictates how the two waves will interact and this is known as the interference.
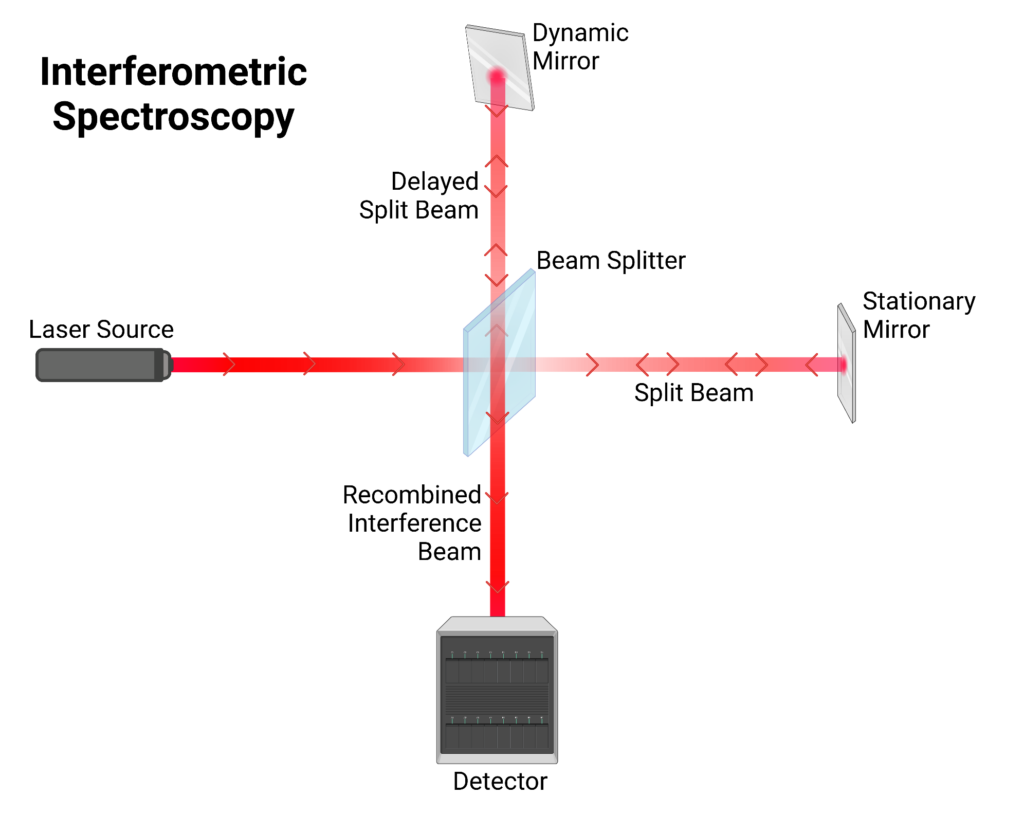
An interferometer measures these interferences. The IR light is directed to a beam splitter which dissects the beam into two, which each go to two mirrors (one stationary and one adjustable), and subsequently bounce back to recombine and interact with the sample for detection. The phase difference is adjusted by moving one of the mirrors which results in a different interference pattern. This difference in interference pattern generates different wavelengths in each recombined beam. The resulting data is plotted in an interferogram.
An interferogram shows how strongly the sample absorbs IR light as a function of the position of the adjustable mirror. This is known as the wavenumber (cm-1): the number of full waves of a particular wavelength per centimeter of length. To produce a spectrum from the interferogram, this data needs to be converted to show how strongly the sample absorbs each frequency of light. This is possible because wavenumbers are directly related to energy levels. A mathematical model called the Fourier-Transform converts this data. It does this by taking the data from wave interference with different frequencies and extracts the frequencies of the original waves. This produces the familiar IR spectrum showing frequency absorption.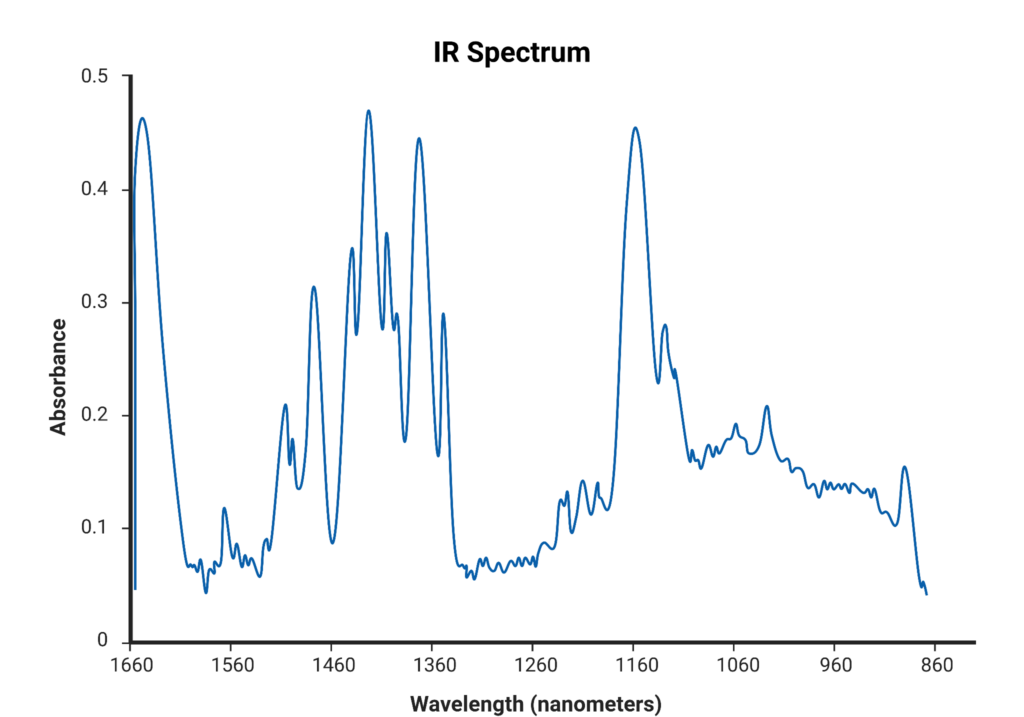 These IR spectra indicate oxidation by showing peaks in the 1800-1670 cm-1 carbonyl (C=O) region which is typically inactive in new lubricants. This is indicative of oxidation because oils are primarily made up of carbon and hydrogen. Hence, the presence of molecular oxygen means chemical processes have occurred to introduce oxygen to the lubricant. These peaks can thus be inferred to be the result of oxidation, and evidence can be given describing the extent of oxidation more extensively than just TAN.
These IR spectra indicate oxidation by showing peaks in the 1800-1670 cm-1 carbonyl (C=O) region which is typically inactive in new lubricants. This is indicative of oxidation because oils are primarily made up of carbon and hydrogen. Hence, the presence of molecular oxygen means chemical processes have occurred to introduce oxygen to the lubricant. These peaks can thus be inferred to be the result of oxidation, and evidence can be given describing the extent of oxidation more extensively than just TAN.
An IR spectrum is also advantageous over titration because it can provide even more information. For example, 600-2000 cm-1 peaks can indicate additives still present in the oil. The presence of nitration products (NO, NO2, and N2O4) is also useful in analyzing degradation progress because nitro-oxides are also prooxidants. These nitro-oxides have a characteristic absorbance just below that of carbonyl products (1650-1600 cm-1). Sulfation products can also indicate degradation and these products are the culprits of the foul smell associated with bad oil. Sulfur is not typically found in lubricant formulations, but it is found in crude oils and may be introduced as additives in fuel or lubricants themselves. Therefore it is not uncommon for sulfur compounds to contaminate or preexist in lubricating oils, and these molecules will also catalyze degradation to produce sulfur di- and tri-oxide (SO2 and SO3). These compounds can also interact with water generated from other byproducts like peroxides to form strong acids like sulfuric acid (H2SO4). These degradation products generate peaks between 1180-1120 cm-1. Both nitration and sulfation products in oil are codegradants to oxidation and give insight into the level of degradation of the oil.
Oil and lubricant systems can be monitored by a variety of other techniques as well. These four analytical techniques are some of the most commonly used monitoring systems.
New Research
Lubricants exist extensively across nearly every industry in some fashion. With the considerable need for lubricating oils, research into extending lubricant lifetimes is a hot topic being investigated worldwide. Some of these research groups are searching for alternatives to petroleum-based lubricants, some are developing new additives for antioxidation and anti-wear amongst other properties, and others are seeking ways to limit the need to replace or even use lubricants in certain systems.
A group based in China (Song, et al. 2024) recently published their ester-based oil anti-aging research on alkylated diphenylamine antioxidant molecular modeling simulations. The group aims to provide a molecular theoretical basis for lubricant oxidation. They combined molecular simulation with experimental methods to determine the anti-aging molecular basis of 4,4′-dimethyldiphenylamine (DMDPA), 4,4′-dioctyldiphenylamine (ODA), and 4,4′-dinonyldiphenylamine (T558) antioxidant activity. They investigated these diphenylamines’ mobility, activity, and diatomic oxygen migration. The molecular simulation results determined that the T558 antioxidant displays better physical resistance to oxidative stress. The experimental results corroborated these simulation results and showed that T558 exhibited better antioxidant properties due to its larger relative molecular mass and longer amine para-alkyl chain. Molecular modeling paired with supportive experimental analyses may be able to determine formulation parameters of lubricants in the future more effectively.
Another interesting recent publication on inerting lubricant systems could provide insight into alternative enclosed operational designs to limit or even eradicate oxidation. The idea to operate a system utilizing a lubricant in an inert atmosphere to limit oxidation was proposed in the 1960s, but without proper technology, this concept was revisited only recently. The results indicate that without the presence of oxygen, oxidative degradation can be severely diminished. Blanketing the operation system in nitrogen could extend the life of in-service lubricants. However, system engineering presents a feasibility problem and more research needs to be done in order to determine the applicability of this project to current systems.
Summary
Lubricants are constantly under attack by oxidizers, but the addition of protective antioxidants helps prolong the life of in-service oils. Molecular oxygen and nitro oxides, contaminants and wear metals, pressure and shear stress, high energy photons including UV light, and notably temperature work in conjunction to expedite the oxidative process of oil. After the process is initiated and free radicals form, the immediate products propagate the cycle, leading to the eventual termination of the cycle only delayed by additives that, once depleted, inevitably signify the end of a lubricant’s lifecycle. The lubricant loses the physical and chemical properties in which it was designed as the oxidative process moves along. The generation of acidic products leads to alterations in the viscosity and lubricity, and the production of heavy molecular weight polymers produces sludge and varnish that disrupt machinery, ultimately inducing clogs and corrosion in the operational system. Composition monitoring of the oil is increasingly important to determine its degree of degradation. It can be done in a variety of ways like visually by analyzing color, chemically by analyzing the acid number, or molecularly by using spectroscopic methods to analyze the overall molecular makeup. New research is constantly being conducted to develop new monitoring methods, manufacture higher-efficiency antioxidants and formulations, and even model new oxidation-inhibiting systems. Oxidative degradation is inevitable in oil systems exposed to the atmosphere, but efforts to understand and diminish its effect on lubricant systems will always be a relevant topic of discussion and research.
Sources
- Anderson International. Oil oxidation: How to measure it and why it matters. Available from: https://www.andersonintl.com/oil-oxidation-how-to-measure-it-and-why-it-matters/
- SKF. Oxidation – the oil killer. Available from: https://www.skf.com/us/products/lubrication-management/recondoil/knowledge-hub/recondoil-articles/oxidation-the-oil-killer
- Machinery Lubrication. Oxidation of lubricant. Available from: https://www.machinerylubrication.com/Read/1028/oxidation-lubricant
- Machinery Lubrication. Oil oxidation stages. Available from: https://www.machinerylubrication.com/Read/30165/oil-oxidation-stages
- UpKeep. Lubricant oxidation effects. Available from: https://upkeep.com/learning/lubricant-oxidation-effects/#:~:text=Lubricant%20oxidation%20is%20a%20gradual,lubrication%20of%20equipment%20and%20machines
- ScienceDirect. Oxidation of hydrocarbons. Available from: https://www.sciencedirect.com/topics/chemistry/oxidation-of-hydrocarbons
- Sandia National Laboratories. Kinetics of hydrocarbon oxidation. Available from: https://crf.sandia.gov/research/chemical-physics/chemical-reactivity/kinetics-of-hydrocarbon-oxidation-clone-2/
- NYE Lubricants. All about additives – the role of antioxidants. Available from: https://www.nyelubricants.com/all-about-additives—the-role-of-antioxidants
- BASF. Antioxidants. Available from: https://automotive-transportation.basf.com/global/en/fuel-and-lubricants/fuel-and-lubricant-solutions/components-for-lubricants/portfolio/antioxidants.html#:~:text=Antioxidants&text=Our%20core%20competency%20in%20antioxidants,and%20prevent%20thermo%2Doxidative%20breakdown
- Spectro Scientific. Measuring oil chemistry: Nitration, oxidation, and sulfation. Available from: https://www.spectrosci.com/knowledge-center/test-parameters/measuring-oil-chemistry-nitration-oxidation-and-sulfation#:~:text=OXIDATION%20%E2%80%94%20Oxidation%20of%20oil%20occurs,severe%20corrosion%20of%20machinery%20parts
- Nature. Mechanism of Fenton reaction. Available from: https://www.nature.com/articles/s41598-020-74646-0#:~:text=Mechanism%20of%20Fenton%20reaction%2C%20which,correction%20for%20density%20functional%20theory
- Byjus. Breakdown of Fenton’s reaction. Available from: https://byjus.com/chemistry/fentons-reaction/#:~:text=by%20Henry%20Fenton.-,Breakdown%20of%20Fenton’s%20Reaction,a%20proton%20as%20the%20byproducts
- Chemistry Europe. One-electron reduction of OER. Available from: [https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/ejoc.202000525#:~:text=The%20one%2Delectron%20reduction%20
- NIH. One electron reduction of O2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9251773/#:~:text=The%20recombination%20(%E2%80%9Cdimerization%E2%80%9D),%E2%80%A2%20reactions%20are%20still%20unknown
- MELScience. Polymerization and polycondensation reactions. Available from: https://melscience.com/MA-en/articles/polymerization-and-polycondensation-reactions/#:~:text=Main%20differences&text=If%20in%20the%20polymerization%20process,original%20composition%20of%20the%20monomer
- EJMOAMS. Different types of antioxidants and their importance. Available from: https://www.ejmoams.com/ejmoams-articles/different-types-of-antioxidants-and-its-importance-84624.html#:~:text=Antioxidants%20by%20their%20mechanism%20are,(3)%20Tertiary%20antioxidants
- ISEL. Prolong equipment life: Keep lubricant oxidation at bay. Available from: https://iselinc.com/prolong-equipment-life-keep-lubricant-oxidation-bay/
- Castrol. Fundamentals of lubrication. Available from: https://thelubricantoracle.castrol.com/HPLguide/page-templates/technical-information
- ScienceDirect. Oil degradation spectroscopy. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0301679X19303597
- Machinery Lubrication. Understanding synthetics and their differences. Available from: https://www.machinerylubrication.com/Read/30161/understanding-synthetics-differences
- Valvoline. Oxidation: Why does your motor oil go bad? Available from: https://www.valvolineglobal.com/en-eur/oxidation-why-does-your-motor-oil-go-bad/
- Precision Lubrication. Antioxidants in lubricants. Available from: https://precisionlubrication.com/articles/antioxidants-in-lubricants/
- Song M, Zhang Y, Chen X, et al. Effect of alkylated diphenylamine antioxidants on the anti-aging properties of ester lubricants at the molecular level: molecular simulations and experiments. Materials Today Communications. 2024;39:109187. doi:10.xxxx/j.mtcomm.
- Zhang J, Wong J, Spikes H. Lubricant inerting—a new era in lubrication technology. Presented at: 24th International Colloquium Tribology; 2024; expert verlag GmbH.





