ASTM D4629
Standard Test Method for Trace Nitrogen in Liquid Petroleum Hydrocarbons by Syringe/Inlet and Oxidative Combustion with Chemiluminescence Detection
ASTM D4629 test method determines the total nitrogen present in liquid hydrocarbons in the concentration range from 0.3 to 100 mg N/kg by combustion and ozone chemiluminescence. Naphthas, oils and fuels boiling from 50 to 400°C, can be analyzed by this test method. Typically, viscosities considered in this method lie between 0.2 and 10 cSt (mm2/s) at room temperature. Samples with viscosities and/or concentrations greater than the scope of the method can be either be diluted with an appropriate solvent or be analyzed in accordance with ASTM D5762 standard. The analyst needs to verify the purity of the solvent and the solubility of the sample in the selected solvent. The possible analytical issues due to incomplete sampling or incomplete combustion of the sample after dilution also must be investigated.
D4629 is the preferred method for the quantification of trace nitrogen in hydrocarbon samples. The controlled and complete combustion of the sample convert all nitrogen bound compounds into nitrogen oxide. The method takes advantage that NO reacts fast and quantitatively with O3 forming an excited NO2* that releases its excess energy in nanoseconds. The released energy is captured by a PMT set at the Red/NIR region. The N-detector response is equimolar and linear with a high dynamic range. The intensity of the energy release is proportional to the original N concentration of the sample. Nitrogen is hardly found in fuels and petroleum products so there are no many product quality specifications involving N, however it is gaining importance due to several factors: It degrades the activity of expensive catalysts, especially platinum based ones used in conversion processes like reforming and isomerization. Also, N at low concentrations competes with S in HDS processes making the desired desulfurization degree hard to achieve, and lastly, N content in aromatics at very low levels are deleterious in the catalytic synthesis of polymers and plasticizers where they are the starting point of these processes. TSHR model TN7000 analyzer possesses all instrumental properties to guarantee precise, reliable and accurate experimental results. Based on these characteristics the system meets with great ease the required precision and accuracy criteria of the method exhibiting an unsurpassed and consistent low LOD”. (Aaron Mendez Ph.D.)
Method Summary:
A measured quantity of the liquid hydrocarbon is sampled by a calibrated syringe and directly injected into the pyrotube of the analyzer at a controlled speed into the vaporization and combustion zones of the instrument. In the inlet zone the sample is transported by an inert stream of a carrier gas, typically Argon or Helium. The hydrocarbon is oxidized in a rich pure oxygen stream in the combustion zone at approximately 1000°C where it is converted into CO2, NO and H2O. After the gases exit the combustion zone they are passed through a semipermeable Nafion® membrane where the water is quantitatively removed. The gases pass through a Mo convertor where NO2 molecules in case they form will be reduced to NO to enhance the detector signal. The gases enter the Nitrogen reaction chamber where they are mixed with a stream of O3 to produced an excited NO2* species. The NO2* molecules upon relaxation release an amount of energy proportional to the N original concentration. The relaxation takes a few nanoseconds and the enrgy which is captured by a Photo Multiplier Tube, is amplified processed and recorded. The signal obtained is proportional to the total nitrogen content of the original sample.
Apparatus
The analyzer of vertical configuration for volatile organic compounds is represented in fig.1. The system is provided with an automatic sample changer and a vaporization inlet system 1; a dual zone combustion furnace 2; the membrane dryer 3; a precautionary particle filter 4; the O3 source and reaction and detector module 5; and the signal acquisition and processing unit 6. 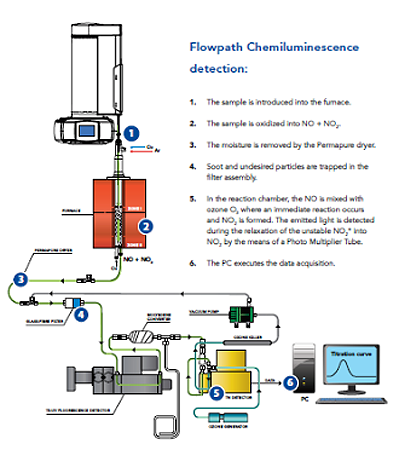 Diagram The nitrogen detector operates at its best at low temperatures, the instrument shown in Fg.1 can operate at temperatures below 0 °C for better sensitivity without causing condensation problems which are frequent in other analyzers.
Diagram The nitrogen detector operates at its best at low temperatures, the instrument shown in Fg.1 can operate at temperatures below 0 °C for better sensitivity without causing condensation problems which are frequent in other analyzers.
Test Procedure:
Repeatability—The difference between two test results obtained by the same operator with the same apparatus under constant operating conditions on identical test material would, in the long run, in the normal and correct operation of the test method, exceed the following values in only once in twenty measurements, where X = the average of the two test results.
r = 0.1825(X) 0.5149
Reproducibility—The difference between two single and independent test results obtained by different operators working in different laboratories on identical test material would, in the long run, in the normal and correct operation of the test method, exceed the following values in only one case in twenty, where X = the average of the two test results.
R = 0.8094(X) 0.5149
Repeatability and Reproducibility
| Concentration (mg/kg S) | r | R |
|---|---|---|
| 100 | 2.0 | 8.7 |
| 75 | 1.7 | 7.5 |
| 50 | 1.4 | 6.1 |
| 25 | 1.0 | 4.2 |
| 10 | 0.6 | 2.6 |
| 1 | 0.18 | 0.81 |
| 0.3 | 0.10 | 0.44 |
Experimental parameters such as sample sizes; flows, and temperatures should be used in accordance with the manufacturer’s operation manual. The same applies to experimental procedures to keep the system in optimum conditions and free of contamination.


